SNARE Complexity in Arbuscular Mycorrhizal Symbiosis
- PMID: 32308661
- PMCID: PMC7145992
- DOI: 10.3389/fpls.2020.00354
SNARE Complexity in Arbuscular Mycorrhizal Symbiosis
Abstract
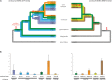
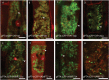
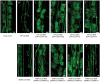
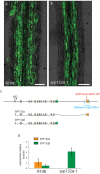
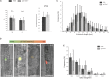
How cells control the proper delivery of vesicles and their associated cargo to specific plasma membrane (PM) domains upon internal or external cues is a major question in plant cell biology. A widely held hypothesis is that expansion of plant exocytotic machinery components, such as SNARE proteins, has led to a diversification of exocytotic membrane trafficking pathways to function in specific biological processes. A key biological process that involves the creation of a specialized PM domain is the formation of a host-microbe interface (the peri-arbuscular membrane) in the symbiosis with arbuscular mycorrhizal fungi. We have previously shown that the ability to intracellularly host AM fungi correlates with the evolutionary expansion of both v- (VAMP721d/e) and t-SNARE (SYP132α) proteins, that are essential for arbuscule formation in Medicago truncatula. Here we studied to what extent the symbiotic SNAREs are different from their non-symbiotic family members and whether symbiotic SNAREs define a distinct symbiotic membrane trafficking pathway. We show that all tested SYP1 family proteins, and most of the non-symbiotic VAMP72 members, are able to complement the defect in arbuscule formation upon knock-down/-out of their symbiotic counterparts when expressed at sufficient levels. This functional redundancy is in line with the ability of all tested v- and t-SNARE combinations to form SNARE complexes. Interestingly, the symbiotic t-SNARE SYP132α appeared to occur less in complex with v-SNAREs compared to the non-symbiotic syntaxins in arbuscule-containing cells. This correlated with a preferential localization of SYP132α to functional branches of partially collapsing arbuscules, while non-symbiotic syntaxins accumulate at the degrading parts. Overexpression of VAMP721e caused a shift in SYP132α localization toward the degrading parts, suggesting an influence on its endocytic turn-over. These data indicate that the symbiotic SNAREs do not selectively interact to define a symbiotic vesicle trafficking pathway, but that symbiotic SNARE complexes are more rapidly disassembled resulting in a preferential localization of SYP132α at functional arbuscule branches.
Keywords: Medicago; SNARE; VAMP; arbuscular mycorrhiza; exocytosis; membrane; symbiosis; syntaxin.
Copyright © 2020 Huisman, Hontelez, Bisseling and Limpens.
Figures


References
LinkOut - more resources
Full Text Sources

