SIRT1-dependent mitochondrial biogenesis supports therapeutic effects of resveratrol against neurodevelopment damage by fluoride
- PMID: 32308752
- PMCID: PMC7163447
- DOI: 10.7150/thno.42387
SIRT1-dependent mitochondrial biogenesis supports therapeutic effects of resveratrol against neurodevelopment damage by fluoride
Abstract
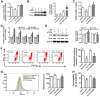
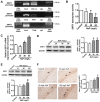
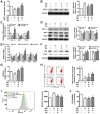
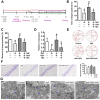
Rationale: Potential adverse effects of fluoride on neurodevelopment has been extensively explored and mitochondria have been recognized as critical targets. Mitochondrial biogenesis serves a crucial role in maintaining mitochondrial homeostasis and salubrious properties of resveratrol (RSV) has been well-defined. However, the molecular mechanisms governing mitochondrial biogenesis in developmental fluoride neurotoxicity remain unclear and the related therapeutic dietary agent is lacking. Methods: In vitro neuroblastoma SH-SY5Y cells and in vivo Sprague-Dawley rat model of developmental fluoride exposure were adopted. A total population of 60 children under long-term stable fluoride exposure were also recruited. This work used a combination of biochemical and behavioral techniques. Biochemical methods included analysis of mitochondrial function and mitochondrial biogenesis, as well as mRNA and protein expression of mitochondrial biogenesis signaling molecules, including silent information regulator 1 (SIRT1), peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM). Behavioral studies investigated spatial learning and memory ability of rats. Results: Both in vivo and in vitro experiments showed that sodium fluoride (NaF) caused mitochondrial dysfunction and impaired mitochondrial biogenesis. Also, NaF elevated SIRT1 levels and suppressed SIRT1 deacetylase activity along with decreased levels of PGC-1α, NRF1 and TFAM, suggestive of dysregulation of mitochondrial biogenesis signaling molecules. Moreover, enhancement of mitochondrial biogenesis by TFAM overexpression alleviated NaF-induced neuronal death through improving mitochondrial function in vitro. Further in vivo and in vitro studies identified RSV, the strongest specific SIRT1 activator, improved mitochondrial biogenesis and subsequent mitochondrial function to protect against developmental fluoride neurotoxicity via activating SIRT1-dependent PGC-1α/NRF1/TFAM signaling pathway. Noteworthy, epidemiological data indicated intimate correlations between disturbed circulating levels of mitochondrial biogenesis signaling molecules and fluoride-caused intellectual loss in children. Conclusions: Our data suggest the pivotal role of impaired mitochondrial biogenesis in developmental fluoride neurotoxicity and the underlying SIRT1 signaling dysfunction in such neurotoxic process, which emphasizes RSV as a potential therapeutic dietary agent for relieving developmental fluoride neurotoxicity.
Keywords: SIRT1; fluoride; mitochondrial biogenesis; neurodevelopmental damage; resveratrol.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
[The effects of resveratrol on mitochondrial biogenesis dysfunction induced by fluoride in human neuroblastoma SH-SY5Y cells].Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2018 Oct 20;36(10):721-727. doi: 10.3760/cma.j.issn.1001-9391.2018.10.001. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2018. PMID: 30541189 Chinese.
-
The protective role of resveratrol on hyperoxia-induced renal injury in neonatal rat by activating the SIRT1/PGC-1α signaling pathway.Eur J Pharmacol. 2025 Apr 15;993:177364. doi: 10.1016/j.ejphar.2025.177364. Epub 2025 Feb 11. Eur J Pharmacol. 2025. PMID: 39947344
-
Resveratrol improved mitochondrial biogenesis by activating SIRT1/PGC-1α signal pathway in SAP.Sci Rep. 2024 Oct 31;14(1):26216. doi: 10.1038/s41598-024-76825-9. Sci Rep. 2024. PMID: 39482340 Free PMC article.
-
Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway.Int Rev Neurobiol. 2019;145:177-209. doi: 10.1016/bs.irn.2019.04.002. Epub 2019 Jun 8. Int Rev Neurobiol. 2019. PMID: 31208524 Free PMC article. Review.
-
Pharmacological approaches to enhance mitochondrial biogenesis: focus on PGC-1Α, AMPK, and SIRT1 in cellular health.Mol Biol Rep. 2025 Feb 28;52(1):270. doi: 10.1007/s11033-025-10368-8. Mol Biol Rep. 2025. PMID: 40019682
Cited by
-
SIRT1: A Potential Therapeutic Target in Autoimmune Diseases.Front Immunol. 2021 Nov 23;12:779177. doi: 10.3389/fimmu.2021.779177. eCollection 2021. Front Immunol. 2021. PMID: 34887866 Free PMC article. Review.
-
Mitochondria, Oxidative Stress, cAMP Signalling and Apoptosis: A Crossroads in Lymphocytes of Multiple Sclerosis, a Possible Role of Nutraceutics.Antioxidants (Basel). 2020 Dec 28;10(1):21. doi: 10.3390/antiox10010021. Antioxidants (Basel). 2020. PMID: 33379309 Free PMC article. Review.
-
Resveratrol's neural protective effects for the injured embryoid body and cerebral organoid.BMC Pharmacol Toxicol. 2022 Jul 12;23(1):47. doi: 10.1186/s40360-022-00593-3. BMC Pharmacol Toxicol. 2022. PMID: 35820950 Free PMC article.
-
TET1 mitigates prenatal fluoride-induced cognition impairment by modulating Bcl2 DNA hydroxymethylation level.Mol Med. 2025 Mar 25;31(1):117. doi: 10.1186/s10020-025-01174-w. Mol Med. 2025. PMID: 40133886 Free PMC article.
-
Ginsenoside Rg3 attenuates neuroinflammation and hippocampal neuronal damage after traumatic brain injury in mice by inactivating the NF-kB pathway via SIRT1 activation.Cell Cycle. 2024 Mar;23(6):662-681. doi: 10.1080/15384101.2024.2355008. Epub 2024 May 25. Cell Cycle. 2024. PMID: 38796716 Free PMC article.
References
-
- Edition F. Guidelines for drinking-water quality. WHO chronicle. 2011;38:104–8. - PubMed
-
- Zhang L, Huang DZ, Yang J, Wei X, Qin J, Ou S. et al. Probabilistic risk assessment of Chinese residents' exposure to fluoride in improved drinking water in endemic fluorosis areas. Environ Pollut. 2017;222:118–25. - PubMed
-
- Khandare AL, Validandi V, Gourineni SR, Gopalan V, Nagalla B. Dose-dependent effect of fluoride on clinical and subclinical indices of fluorosis in school going children and its mitigation by supply of safe drinking water for 5 years: an Indian study. Environ Monit Assess. 2018;190:110. - PubMed
-
- Yousefi M, Ghoochani M, Hossein Mahvi A. Health risk assessment to fluoride in drinking water of rural residents living in the Poldasht city, Northwest of Iran. Ecotoxicol Environ Saf. 2018;148:426–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

