Ultrasmall gold nanoparticles in cancer diagnosis and therapy
- PMID: 32308760
- PMCID: PMC7163431
- DOI: 10.7150/thno.42471
Ultrasmall gold nanoparticles in cancer diagnosis and therapy
Abstract
Due to their lower systemic toxicity, faster kidney clearance and higher tumor accumulation, ultrasmall gold nanoparticles (less than 10 nm in diameter) have been proved to be promising in biomedical applications. However, their potential applications in cancer imaging and treatment have not been reviewed yet. This review summarizes the efforts to develop systems based on ultrasmall gold nanoparticles for use in cancer diagnosis and therapy. First, we describe the methods for controlling the size and surface functionalization of ultrasmall gold nanoparticles. Second, we review the research on ultrasmall gold nanoparticles in cancer imaging and treatment. Specifically, we focus on the applications of ultrasmall gold nanoparticles in tumor visualization and bioimaging in different fields such as magnetic resonance imaging, photoacoustic imaging, fluorescence imaging, and X-ray scatter imaging. We also highlight the applications of ultrasmall gold nanoparticles in tumor chemotherapy, radiotherapy, photodynamic therapy and gene therapy.
Keywords: cancer therapy; imaging; size; theranostics; ultrasmall gold nanoparticles.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
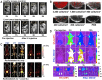

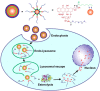
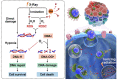
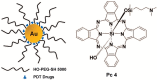




References
-
- Kumar A, Mazinder Boruah B, Liang XJ. Gold nanoparticles: promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J Nanomater. 2011;2011:1–17.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

