An Open-label, Single-center, Safety and Efficacy Study of Eyelash Polygrowth Factor Serum
- PMID: 32308787
- PMCID: PMC7158911
An Open-label, Single-center, Safety and Efficacy Study of Eyelash Polygrowth Factor Serum
Abstract
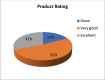
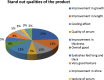
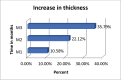
Objective: We sought to evaluate the efficacy of a polygrowth factor serum for increasing length, luster, thickness, and volume of eyelashes in a group of healthy Indian women. Design: This was a 90-day, open-label, single-center safety and efficacy study. Thirty Indian female participants, aged 15 to 45 years old, were enrolled in the study; 29 of these subjects completed the study. There were four assessment visits: at baseline (Day 0) and on Days 30, 60, and 90. Subjects were instructed on the application of the test product uniformly to both eyes on the upper and lower eyelid margins. Subjects applied the product once nightly for 90 days. Measurements: At each visit, subjects underwent ophthalmological and dermatological assessments and digital image photographs using Visia CR imaging system. Improvement in eyelash length, density/volume, luster, and curl were evaluated using imaging and software technologies. Results: Improvement in test parameters was observed 30 days after initiation of product usage. Among the 29 subjects who completed the study, improvements in length (10.52%), volume (9.3%), luster (11.43%), thickness (35%), and curl (50.83%), compared to baseline, were recorded. Conclusion: The study demonstrated efficacy in improving eyelash length, luster, thickness, and curl. The results were observed as early as 30 days of product usage and persisted until the last visit following 90 days of product usage. There were no adverse events associated with the product. We concluded that the polygrowth factor serum was well tolerated and effectively improved eye lash length, luster, thickness, and volume in our patient sample. Additional randomized, controlled studies with larger samples are needed to confirm our findings.
Keywords: Polygrowth factor serum; eyelash growth.
Copyright © 2020. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:This study was funded by an educational grant from SkinGen International Inc. DISCLOSURES:The authors have no conflicts of interest relevant to the content of this article.
Figures










References
LinkOut - more resources
Full Text Sources
Medical
