Postmarket Modifications of High-risk Plastic Surgery Devices
- PMID: 32309074
- PMCID: PMC7159931
- DOI: 10.1097/GOX.0000000000002621
Postmarket Modifications of High-risk Plastic Surgery Devices
Abstract
Background: In the United States, high-risk medical devices must be cleared through the premarket approval (PMA) pathway, which requires clinical evidence ensuring safety and efficacy. Approved devices can be modified and reintroduced to market without additional study through the PMA supplemental review track. This study characterizes the changes of high-risk plastic surgery devices once they undergo initial clearance.
Methods: A retrospective, cross-sectional analysis of the Food and Drug Administration (FDA) PMA database. The following data were extracted from the PMA database (January 1, 1980 to December 31, 2018): initial clearance date, device type, the number and type of supplement, supplement reason, and product withdrawal date. Data from the FDA medical device recall database were also extracted and reported. The median number of device modifications and median lifetime of device-years were calculated.
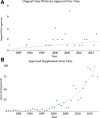
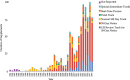
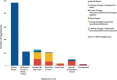
Results: There have been 39 original plastic surgery devices approved by the FDA. There was no significant change with respect to initial clearance dates for original devices over time (r = 0.28; P = 0.084). PMA supplement usage has significantly increased with time (rs = 0.9174, P = 0.000). Overall, approved plastic surgery devices have undergone a median of 11 changes (IQR, 3-35). Breast implant devices collectively underwent the most modifications with a median of 28 modifications per device (IQR, 20.25-33.25).
Conclusions: Over the past 2 decades, plastic surgery device manufacturers have significantly increased the use of supplement track review. High-risk plastic surgery devices may undergo frequent minor changes without clinical evidence to support the safety and efficacy of modified versions.
Copyright © 2020 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of The American Society of Plastic Surgeons.
Figures
References
-
- Medical devices 2030. https://advisory.kpmg.us/articles/2018/medical-devices-2030.html. Accessed March 29, 2019.
-
- U.S. Department of Health and Human Services. Regulated Products – Medical Device Overview. https://www.fda.gov/forindustry/importprogram/importbasics/regulatedprod.... Accessed April 14, 2019.
-
- Faris O, Shuren J. An FDA viewpoint on unique considerations for medical-device clinical trials. N Engl J Med. 2017;376:1350–1357. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous