Treatment of Renal Anemia with Roxadustat: Advantages and Achievement
- PMID: 32309288
- PMCID: PMC7154284
- DOI: 10.1159/000504850
Treatment of Renal Anemia with Roxadustat: Advantages and Achievement
Abstract
Background: Although renal anemia has attracted widespread attention, a large proportion of chronic kidney disease (CKD) patients with anemia still do not meet the hemoglobin (Hb) targets. The discovery of prolyl hydroxylase domain (PHD) enzymes as regulators of hypoxia-inducible factor (HIF)-dependent erythropoiesis has led to the development of novel therapeutic agents for renal anemia. Roxadustat, the first small-molecule HIF-PHD inhibitor, has completed the phase 3 trials. There are currently more than 15 phase 3 clinical trials worldwide assessing the efficacy and safety of roxadustat in CKD patients with anemia. This review will summarize recent findings of roxadustat in the treatment of renal anemia.
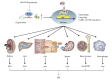
Summary: Although the administration of erythropoiesis-stimulating agents (ESAs) and iron supplementation are a well-established and highly effective therapeutic approach for renal anemia, there are several safety concerns. Current findings from phase 2 and 3 trials suggest that roxadustat is clinically effective and well tolerated. On the one hand, roxadustat could increase endogenous erythropoietin (EPO) levels within or near physiological range in a titratable manner by inducing HIF pathway activation transiently. On the other hand, roxadustat also improves iron metabolism by decreasing serum hepcidin and increasing intestinal iron absorption, which is beneficial to functional iron deficiency and absolute iron deficiency. More importantly, the erythropoietic response of roxadustat is independent of baseline inflammatory state of CKD patients. Thus, the discovery of roxadustat will revolutionize the treatment strategy for renal anemia.
Key messages: Roxadustat is an emerging and promising therapeutic approach against anemia in CKD patients, which differs from those of conventional ESAs. Roxadustat corrects anemia of CKD patients through multiple pathways, beyond elevating EPO levels within physiological range, and also by handling iron metabolism (particularly decreasing the hepcidin levels). Furthermore, the Hb response of roxadustat is independent of the inflammatory microenvironment.
Keywords: Erythropoietin; Hepcidin; Iron metabolism; Renal anemia; Roxadustat.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

