Association of survival and genomic mutation signature with immunotherapy in patients with hepatocellular carcinoma
- PMID: 32309377
- PMCID: PMC7154492
- DOI: 10.21037/atm.2020.01.32
Association of survival and genomic mutation signature with immunotherapy in patients with hepatocellular carcinoma
Abstract
Background: Current guidelines lack recommendations for the use of immunotherapy and immune-related biomarkers for hepatocellular carcinoma (HCC). We aim to provide reliable evidence of the association of survival with HCC immunotherapy and to demonstrate that genomic mutation signature could be an effective biomarker to predict immunotherapy efficacy of HCC patients.
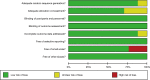
Methods: We conducted a meta-analysis of 17 randomized trials with 2055 patients and an individual patient-level analysis of 31 patients. Trial data were identified in PubMed, EMBASE and Cochrane Central library, and individual patient data were obtained from the cBioPortal database. Overall survival (OS) and progression-free survival (PFS) were assessed with the hazard ratio (HR) and 95% CI. This study is registered with PROSPERO, number CRD42018083991.
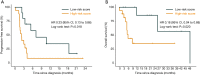
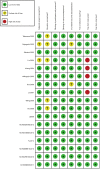
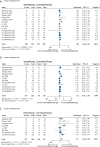
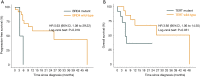
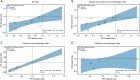
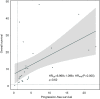
Results: The meta-analysis showed that compared to conventional therapy, immunotherapy resulted in prolonged OS (HR =0.65, P<0.0001, high quality) and PFS (HR =0.81, P<0.0001, high quality); the benefits were observed for cellular immunotherapy, tumor vaccine, and cytokine immunotherapy. Findings were robust to subgroup and trial sequential analyses. In the individual patient-level analysis of patients treated with immune checkpoint inhibitor, mutations in TERT, CTNNB1, BRD4, or MLL, and co-mutations in TP53 and TERT or BRD4 were associated with significantly worse survival. These oncogenes were used to develop a novel integrated mutation risk score, which exhibited better utility in predicting survival than the tumor mutation burden (TMB). Patients with low- versus high- mutation risk score had longer OS (HR =0.18, P=0.02) and PFS (HR =0.33, P=0.018). A nomogram comprising the mutation risk score and essential clinical factors further improved the predictive accuracy (AUC =0.840 for both 1- and 2-year OS).
Conclusions: Immunotherapy showed longer OS and PFS than conventional therapy among HCC patients, especially patients with a low mutation risk score. The nomogram based on genomic and clinical characteristics is effective in predicting survival of HCC patients undergoing immune checkpoint inhibitor.
Keywords: Hepatocellular carcinoma (HCC); efficacy; genomic mutation; immunotherapy.
2020 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures











Similar articles
-
Association of Survival and Immune-Related Biomarkers With Immunotherapy in Patients With Non-Small Cell Lung Cancer: A Meta-analysis and Individual Patient-Level Analysis.JAMA Netw Open. 2019 Jul 3;2(7):e196879. doi: 10.1001/jamanetworkopen.2019.6879. JAMA Netw Open. 2019. PMID: 31290993 Free PMC article.
-
Combination of alpha-fetoprotein and neutrophil-to-lymphocyte ratio to predict treatment response and survival outcomes of patients with unresectable hepatocellular carcinoma treated with immune checkpoint inhibitors.BMC Cancer. 2023 Jun 15;23(1):547. doi: 10.1186/s12885-023-11003-0. BMC Cancer. 2023. PMID: 37322411 Free PMC article.
-
CTNNB1 Alternation Is a Potential Biomarker for Immunotherapy Prognosis in Patients With Hepatocellular Carcinoma.Front Immunol. 2021 Oct 28;12:759565. doi: 10.3389/fimmu.2021.759565. eCollection 2021. Front Immunol. 2021. PMID: 34777372 Free PMC article.
-
Predictive value of tumor mutational burden for immunotherapy in non-small cell lung cancer: A systematic review and meta-analysis.PLoS One. 2022 Feb 3;17(2):e0263629. doi: 10.1371/journal.pone.0263629. eCollection 2022. PLoS One. 2022. PMID: 35113949 Free PMC article.
-
Predictive Efficacy of Blood-Based Tumor Mutation Burden Assay for Immune Checkpoint Inhibitors Therapy in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis.Front Oncol. 2022 Feb 9;12:795933. doi: 10.3389/fonc.2022.795933. eCollection 2022. Front Oncol. 2022. PMID: 35223476 Free PMC article.
Cited by
-
Downregulated Ferroptosis-Related Gene STEAP3 as a Novel Diagnostic and Prognostic Target for Hepatocellular Carcinoma and Its Roles in Immune Regulation.Front Cell Dev Biol. 2021 Nov 1;9:743046. doi: 10.3389/fcell.2021.743046. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34790664 Free PMC article.
-
Analysis of Multi-Layer RNA Modification Patterns for the Characterization of Tumor Immune Microenvironment in Hepatocellular Carcinoma.Front Cell Dev Biol. 2021 Nov 10;9:761391. doi: 10.3389/fcell.2021.761391. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34858985 Free PMC article.
-
Prognostic genome and transcriptome signatures in colorectal cancers.Nature. 2024 Sep;633(8028):137-146. doi: 10.1038/s41586-024-07769-3. Epub 2024 Aug 7. Nature. 2024. PMID: 39112715 Free PMC article.
-
Prognostic model of immune checkpoint inhibitors combined with anti-angiogenic agents in unresectable hepatocellular carcinoma.Front Immunol. 2022 Dec 1;13:1060051. doi: 10.3389/fimmu.2022.1060051. eCollection 2022. Front Immunol. 2022. PMID: 36532029 Free PMC article.
-
Beta-catenin activation and immunotherapy resistance in hepatocellular carcinoma: mechanisms and biomarkers.Hepatoma Res. 2021;7:8. doi: 10.20517/2394-5079.2020.124. Epub 2021 Jan 7. Hepatoma Res. 2021. PMID: 33553649 Free PMC article.
References
-
- ClinicalTrialgov. Transarterial Chemoembolization in Combination With Nivolumab Performed for Intermediate Stage Hepatocellular Carcinoma (IMMUTACE) Available online: https://clinicaltrialsgov/ct2/show/NCT03572582 (accessed on 31 June 2019).
-
- Harding JJ, Erinjeri JP, Tan BR, et al. A multicenter pilot study of nivolumab (NIVO) with drug eluting bead transarterial chemoembolization (deb-TACE) in patients (pts) with liver limited hepatocellular carcinoma (HCC). J Clin Oncol 2018;36:TPS4146. 10.1200/JCO.2018.36.15_suppl.TPS4146 - DOI
-
- Kaseb A, Vence L, Blando J, et al. Randomized, open-label, perioperative phase II study evaluating nivolumab alone versus nivolumab plus ipilimumab in patients with resectable HCC. Ann Oncol 2019;30 Suppl 4:iv112. 10.1093/annonc/mdz156.008 - DOI
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
