Functional Amyloids and their Possible Influence on Alzheimer Disease
- PMID: 32309597
- PMCID: PMC7159844
- DOI: 10.15190/d.2017.9
Functional Amyloids and their Possible Influence on Alzheimer Disease
Abstract
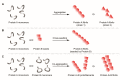
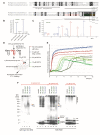
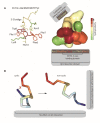
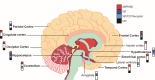
Amyloids play critical roles in human diseases but have increasingly been recognized to also exist naturally. Shared physicochemical characteristics of amyloids and of their smaller oligomeric building blocks offer the prospect of molecular interactions and crosstalk amongst these assemblies, including the propensity to mutually influence aggregation. A case in point might be the recent discovery of an interaction between the amyloid β peptide (Aβ) and somatostatin (SST). Whereas Aβ is best known for its role in Alzheimer disease (AD) as the main constituent of amyloid plaques, SST is intermittently stored in amyloid-form in dense core granules before its regulated release into the synaptic cleft. This review was written to introduce to readers a large body of literature that surrounds these two peptides. After introducing general concepts and recent progress related to our understanding of amyloids and their aggregation, the review focuses separately on the biogenesis and interactions of Aβ and SST, before attempting to assess the likelihood of encounters of the two peptides in the brain, and summarizing key observations linking SST to the pathobiology of AD. While the review focuses on Aβ and SST, it is to be anticipated that crosstalk amongst functional and disease-associated amyloids will emerge as a general theme with much broader significance in the etiology of dementias and other amyloidosis.
Keywords: Alzheimer disease; Aβ; amyloid; interaction; oligomeric; somatostatin.
Copyright: © 2017, Lau et al. and Applied Systems.
Conflict of interest statement
Conflict of interests: A provisional US patent (application number 62/451-309) titled ‘Oligomeric Abeta-Binding Polypeptides) that lists HW and GS as inventors was filed on January 27, 2017, by the University of Toronto.
Figures

References
-
- The amyloid state and its association with protein misfolding diseases. Knowles Tuomas P. J., Vendruscolo Michele, Dobson Christopher M. Nature Reviews Molecular Cell Biology. 2014;15(6):384-396. - PubMed
-
- Common Structure of Soluble Amyloid Oligomers Implies Common Mechanism of Pathogenesis. Kayed R. Science. 2003;300(5618):486-489. - PubMed
-
- Prefibrillar Amyloid Protein Aggregates Share Common Features of Cytotoxicity. Bucciantini Monica, Calloni Giulia, Chiti Fabrizio, Formigli Lucia, Nosi Daniele, Dobson Christopher M., Stefani Massimo. Journal of Biological Chemistry. 2004;279(30):31374-31382. - PubMed
-
- Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Glenner George G., Wong Caine W. Biochemical and Biophysical Research Communications. 1984;120(3):885-890. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
