An Aminotransferase from Enhydrobacter aerosaccus to Obtain Optically Pure β-Phenylalanine
- PMID: 32309682
- PMCID: PMC7160847
- DOI: 10.1021/acsomega.9b03416
An Aminotransferase from Enhydrobacter aerosaccus to Obtain Optically Pure β-Phenylalanine
Abstract
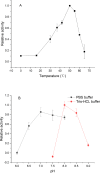
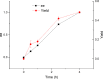
An aminotransferase ω-TAEn was identified from Enhydrobacter aerosaccus. The ω-TAEn was successfully expressed in Escherichia coli and the obtained enzyme showed activity toward β-phenylalanine (β-phe) at optimal conditions. For optically pure (R)-β-phe, 50% yield was observed by kinetic resolution of racemic amino with pyruvate as the amino acceptor. To obtain (S)-β-phe, the lipase/ω-TAEn catalytic system was adopted. The ω-TAEn showed strict stereoselectivity to the amino donor. The formation of (S)-β-phe was observed using 3-aminobutyric acid as the amino donor, and (S)-β-phe was obtained by asymmetric synthesis with a yield of 82%.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Savile C. K.; Janey J. M.; Mundorff E. C.; Moore J. C.; Tam S.; Jarvis W. R.; Colbeck J. C.; Krebber A.; Fleitz F. J.; Brands J.; Devine P. N.; Huisman G. W.; Hughes G. J. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. 10.1126/science.1188934. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
