Myeloid-specific Asxl2 deletion limits diet-induced obesity by regulating energy expenditure
- PMID: 32310225
- PMCID: PMC7190927
- DOI: 10.1172/JCI128687
Myeloid-specific Asxl2 deletion limits diet-induced obesity by regulating energy expenditure
Abstract
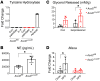
We previously established that global deletion of the enhancer of trithorax and polycomb (ETP) gene, Asxl2, prevents weight gain. Because proinflammatory macrophages recruited to adipose tissue are central to the metabolic complications of obesity, we explored the role of ASXL2 in myeloid lineage cells. Unexpectedly, mice without Asxl2 only in myeloid cells (Asxl2ΔLysM) were completely resistant to diet-induced weight gain and metabolically normal despite increased food intake, comparable activity, and equivalent fecal fat. Asxl2ΔLysM mice resisted HFD-induced adipose tissue macrophage infiltration and inflammatory cytokine gene expression. Energy expenditure and brown adipose tissue metabolism in Asxl2ΔLysM mice were protected from the suppressive effects of HFD, a phenomenon associated with relatively increased catecholamines likely due to their suppressed degradation by macrophages. White adipose tissue of HFD-fed Asxl2ΔLysM mice also exhibited none of the pathological remodeling extant in their control counterparts. Suppression of macrophage Asxl2 expression, via nanoparticle-based siRNA delivery, prevented HFD-induced obesity. Thus, ASXL2 controlled the response of macrophages to dietary factors to regulate metabolic homeostasis, suggesting modulation of the cells' inflammatory phenotype may impact obesity and its complications.
Keywords: Adipose tissue; Macrophages; Metabolism; Obesity.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK060022/DK/NIDDK NIH HHS/United States
- R01 AR046523/AR/NIAMS NIH HHS/United States
- R37 AR046523/AR/NIAMS NIH HHS/United States
- R01 AR068972/AR/NIAMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- U24 CA209837/CA/NCI NIH HHS/United States
- R01 DK111389/DK/NIDDK NIH HHS/United States
- R01 HL073646/HL/NHLBI NIH HHS/United States
- R01 AR064755/AR/NIAMS NIH HHS/United States
- R00 HL138163/HL/NHLBI NIH HHS/United States
- P41 EB025815/EB/NIBIB NIH HHS/United States
- K99 HL138163/HL/NHLBI NIH HHS/United States
- R01 DK102691/DK/NIDDK NIH HHS/United States
- P30 AR074992/AR/NIAMS NIH HHS/United States
- R01 DK119437/DK/NIDDK NIH HHS/United States
- DP1 DK109668/DK/NIDDK NIH HHS/United States
- R01 HL038180/HL/NHLBI NIH HHS/United States
- R37 HL038180/HL/NHLBI NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- R01 DK056260/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- R01 AR070975/AR/NIAMS NIH HHS/United States
- DP5 OD028125/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

