GDF15: A Hormone Conveying Somatic Distress to the Brain
- PMID: 32310257
- PMCID: PMC7299427
- DOI: 10.1210/endrev/bnaa007
GDF15: A Hormone Conveying Somatic Distress to the Brain
Abstract
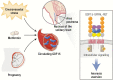
GDF15 has recently gained scientific and translational prominence with the discovery that its receptor is a GFRAL-RET heterodimer of which GFRAL is expressed solely in the hindbrain. Activation of this receptor results in reduced food intake and loss of body weight and is perceived and recalled by animals as aversive. This information encourages a revised interpretation of the large body of previous research on the protein. GDF15 can be secreted by a wide variety of cell types in response to a broad range of stressors. We propose that central sensing of GDF15 via GFRAL-RET activation results in behaviors that facilitate the reduction of exposure to a noxious stimulus. The human trophoblast appears to have hijacked this signal, producing large amounts of GDF15 from early pregnancy. We speculate that this encourages avoidance of potential teratogens in pregnancy. Circulating GDF15 levels are elevated in a range of human disease states, including various forms of cachexia, and GDF15-GFRAL antagonism is emerging as a therapeutic strategy for anorexia/cachexia syndromes. Metformin elevates circulating GDF15 chronically in humans and the weight loss caused by this drug appears to be dependent on the rise in GDF15. This supports the concept that chronic activation of the GDF15-GFRAL axis has efficacy as an antiobesity agent. In this review, we examine the science of GDF15 since its identification in 1997 with our interpretation of this body of work now being assisted by a clear understanding of its highly selective central site of action.
Keywords: GDF15; GFRAL; RET; cachexia; hyperemesis gravidarum; obesity.
© Endocrine Society 2020.
Figures



References
-
- Lawton LN, Bonaldo MF, Jelenc PC, et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene. 1997;203(1):17–26. - PubMed
-
- Böttner M, Suter-Crazzolara C, Schober A, Unsicker K. Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell Tissue Res. 1999;297(1):103–110. - PubMed
-
- Paralkar VM, Vail AL, Grasser WA, et al. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J Biol Chem. 1998;273(22):13760–13767. - PubMed
-
- Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L. PLAB, a novel placental bone morphogenetic protein. Biochim Biophys Acta. 1997;1354(1):40–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

