Deficit of circulating CD19+ CD24hi CD38hi regulatory B cells in severe aplastic anaemia
- PMID: 32311088
- PMCID: PMC7496711
- DOI: 10.1111/bjh.16651
Deficit of circulating CD19+ CD24hi CD38hi regulatory B cells in severe aplastic anaemia
Abstract
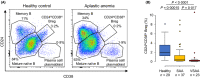
Immune aplastic anaemia (AA) is caused by cytotoxic T lymphocytes (CTLs) that destroy haematopoietic stem and progenitor cells. Enhanced type 1 T helper (Th1) responses and reduced regulatory T cells (Tregs) are involved in the immune pathophysiology. CD24hi CD38hi regulatory B cells (Bregs) suppress CTLs and Th1 responses, and induce Tregs via interleukin 10 (IL-10). We investigated circulating B-cell subpopulations, including CD24hi CD38hi Bregs, as well as total B cells, CD4+ T cells, CD8+ T cells and natural killer cells in 104 untreated patients with severe and very severe AA, aged ≥18 years. All patients were treated with standard immunosuppressive therapy (IST) plus eltrombopag. CD24hi CD38hi Bregs were markedly reduced in patients with AA compared to healthy individuals, especially in very severe AA, but residual Bregs remained functional, capable of producing IL-10; total B-cell counts and the other B-cell subpopulations were similar to those of healthy individuals. CD24hi CD38hi Bregs did not correlate with responses to IST, and they recovered to levels present in healthy individuals after therapy. Mature naïve B-cell counts were unexpectedly associated with IST response. Markedly reduced CD24hi CD38hi Bregs, especially in very severe AA, with recovery after IST suggest Breg deficits may contribute to the pathophysiology of immune AA.
Keywords: aplastic anaemia; flow cytometry; immunosuppressive therapy; lymphocyte subsets; regulatory B cells.
Published 2020. This article is a U.S. Government work and is in the public domain in the USA. British Journal of Haematology published by British Society of Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


Comment in
-
Regulatory cells in immune-mediated aplastic anaemia - not Tregs but Bregs.Br J Haematol. 2020 Aug;190(4):486-487. doi: 10.1111/bjh.16713. Epub 2020 Jun 4. Br J Haematol. 2020. PMID: 32583450 No abstract available.
References
-
- Katagiri T, Sato‐Otsubo A, Kashiwase K, Morishima S, Sato Y, Mori Y, et al. Frequent loss of HLA alleles associated with copy number‐neutral 6pLOH in acquired aplastic anemia. Blood. 2011;118:6601–9. - PubMed
-
- Zaimoku Y, Takamatsu H, Hosomichi K, Ozawa T, Nakagawa N, Imi T, et al. Identification of an HLA class I allele closely involved in the autoantigen presentation in acquired aplastic anemia. Blood. 2017;129:2908–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

