Pentathiepins: A Novel Class of Glutathione Peroxidase 1 Inhibitors that Induce Oxidative Stress, Loss of Mitochondrial Membrane Potential and Apoptosis in Human Cancer Cells
- PMID: 32311219
- PMCID: PMC7496275
- DOI: 10.1002/cmdc.202000160
Pentathiepins: A Novel Class of Glutathione Peroxidase 1 Inhibitors that Induce Oxidative Stress, Loss of Mitochondrial Membrane Potential and Apoptosis in Human Cancer Cells
Abstract
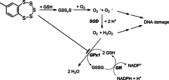
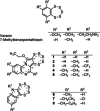
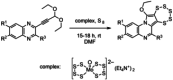
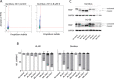
A novel class of glutathione peroxidase 1 (GPx1) inhibitors, namely tri- and tetracyclic pentathiepins, has been identified that is approximately 15 times more potent than the most active known GPx1 inhibitor, mercaptosuccinic acid. Enzyme kinetic studies with bovine erythrocyte GPx1 indicate that pentathiepins reversibly inhibit oxidation of the substrate glutathione (GSH). Moreover, no inhibition of superoxide dismutase, catalase, thioredoxin reductase or glutathione reductase was observed at concentrations that effectively inhibit GPx1. As well as potent enzyme inhibitory activity, the pentathiepins show strong anticancer activity in various human cancer cell lines, with IC50 values in a low-micromolar range. A representative tetracyclic pentathiepin causes the formation of reactive oxygen species in these cells, the fragmentation of nuclear DNA and induces apoptosis via the intrinsic pathway. Moreover, this pentathiepin leads to a rapid and strong loss of mitochondrial membrane potential in treated cancer cells. On the other hand, evidence for the induction of ferroptosis as a form of cell death was negative. These new findings show that pentathiepins possess interesting biological activities beyond those originally ascribed to these compounds.
Keywords: DNA fragmentation; apoptosis; cancer cells; cytotoxicity; glutathione peroxidase; oxidative stress; pentathiepin.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rocher C., Lalanne J. L., Chaudiere J., Eur. J. Biochem. 1992, 205, 955–960. - PubMed
-
- Toppo S., Flohe L., Ursini F., Vanin S., Maiorino M., Biochim. Biophys. Acta 2009, 1790, 1486–1500. - PubMed
-
- None
-
- Ho Y. S., Magnenat J. L., Bronson R. T., Cao J., Gargano M., Sugawara M., Funk C. D., J. Biol. Chem. 1997, 272, 16644–16651; - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

