Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy
- PMID: 32311700
- PMCID: PMC8715366
- DOI: 10.1038/s41390-020-0885-7
Effects of vitamin D supplementation on circulating concentrations of growth factors and immune-mediators in healthy women during pregnancy
Abstract
Background: For the second aim of the Kellogg Foundation grant, this double-blind RCT investigated the impact of plasma vitamin D metabolite 25-hydroxyvitamin D (25(OH)D) on plasma immune-mediators during pregnancy. We hypothesized that higher 25(OH)D concentrations would associate with reduced pro-inflammatory and increased tolerogenic immune-mediator concentrations.
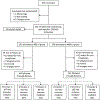
Methods: Pregnant women enrolled at 10-14 weeks gestation were randomized to 400 or 4400 IU vitamin D3/day. Data on health, safety, circulating 25(OH)D, and 9 immune-mediators were collected at each trimester. Associations between immune-mediators and 25(OH)D at baseline and at second and third trimesters were examined.
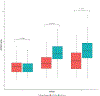
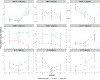
Results: Baseline TGF-β and second and third trimesters IFN-γ and IL-2 were associated with baseline 25(OH)D. Baseline immune-mediators were associated with immune-mediators at second and third trimesters for all immune-mediators except IL-5 and IL-10. Race was associated with baseline TGF-β, VEGF and IL-10 and with IL-10 at second and third trimesters.
Conclusions: Both treatment groups had increased 25(OH)D at second and third trimesters, greatest in the 4400 IU group. Though associations between baseline 25(OH)D and baseline TGF-β and second and third trimester IFN-γ and IL-2 were noted, vitamin D supplementation throughout pregnancy did not impact immune-mediators at later trimesters. Supplementing with vitamin D before conception conceivably influences immune-mediator responses during pregnancy.
Impact: In this vitamin D supplementation clinical trial, baseline (first trimester) but not increasing plasma 25(OH)D concentration impacted select plasma immune-mediator profiles in pregnant women. Baseline 25(OH)D was associated with baseline TGF-β and with IFN-γ and IL-2 at second and third trimesters. Baseline IFN-γ, CRP, TGF-β, TNF-α, VEGF, IL-2, and IL-4 were associated with concentrations at second and third trimesters for respective immune-mediators; however, 25(OH)D concentration at second and third trimesters were not. Some racial differences existed in immune-mediator concentrations at baseline and at second and third trimesters. This study assesses the impact of vitamin D supplementation on multiple immune-mediators in pregnant women of different racial/ethnic groups using longitudinal data from a relatively large randomized controlled trial. This study found that race was associated with baseline TGF-β, VEGF, and IL-10 and with IL-10 at second and third trimesters, a novel finding that sheds light where relationships were less well defined. The results of this study suggest that vitamin D supplementation before conception or early in pregnancy, rather than during pregnancy, may be necessary to significantly impact immune-mediator response. This study sets premise for future clinical trials to evaluate the effect of vitamin D supplementation before conception or prior to pregnancy.
Conflict of interest statement
Figures
Similar articles
-
Randomized placebo-controlled trial of high-dose prenatal third-trimester vitamin D3 supplementation in Bangladesh: the AViDD trial.Nutr J. 2013 Apr 12;12:47. doi: 10.1186/1475-2891-12-47. Nutr J. 2013. PMID: 23587190 Free PMC article. Clinical Trial.
-
Randomized trial of three doses of vitamin D to reduce deficiency in pregnant Mongolian women.EBioMedicine. 2019 Jan;39:510-519. doi: 10.1016/j.ebiom.2018.11.060. Epub 2018 Dec 11. EBioMedicine. 2019. PMID: 30552064 Free PMC article. Clinical Trial.
-
Determinants of the Maternal 25-Hydroxyvitamin D Response to Vitamin D Supplementation During Pregnancy.J Clin Endocrinol Metab. 2016 Dec;101(12):5012-5020. doi: 10.1210/jc.2016-2869. Epub 2016 Oct 27. J Clin Endocrinol Metab. 2016. PMID: 27788053 Free PMC article. Clinical Trial.
-
Early-Life Effects of Vitamin D: A Focus on Pregnancy and Lactation.Ann Nutr Metab. 2020;76 Suppl 2:16-28. doi: 10.1159/000508422. Epub 2020 Nov 24. Ann Nutr Metab. 2020. PMID: 33232956 Review.
-
Effects of vitamin D3 supplementation on serum 25(OH)D concentration and strength in athletes: a systematic review and meta-analysis of randomized controlled trials.J Int Soc Sports Nutr. 2019 Nov 26;16(1):55. doi: 10.1186/s12970-019-0323-6. J Int Soc Sports Nutr. 2019. PMID: 31771586 Free PMC article.
Cited by
-
Substantial Vitamin D Supplementation Is Required during the Prenatal Period to Improve Birth Outcomes.Nutrients. 2022 Feb 21;14(4):899. doi: 10.3390/nu14040899. Nutrients. 2022. PMID: 35215549 Free PMC article.
-
The effect of vitamin D supplementation on oxidative stress and inflammatory biomarkers in pregnant women: a systematic review and meta-analysis of clinical trials.BMC Pregnancy Childbirth. 2022 Nov 5;22(1):816. doi: 10.1186/s12884-022-05132-w. BMC Pregnancy Childbirth. 2022. PMID: 36335311 Free PMC article.
-
Do Micronutrient and Omega-3 Fatty Acid Supplements Affect Human Maternal Immunity during Pregnancy? A Scoping Review.Nutrients. 2022 Jan 15;14(2):367. doi: 10.3390/nu14020367. Nutrients. 2022. PMID: 35057548 Free PMC article.
-
Does maternal vitamin D status influence placental weight or vascular and inflammatory pathology? Secondary analysis from the Kellogg Pregnancy Study.J Steroid Biochem Mol Biol. 2023 Oct;233:106358. doi: 10.1016/j.jsbmb.2023.106358. Epub 2023 Jul 4. J Steroid Biochem Mol Biol. 2023. PMID: 37414103 Free PMC article. Clinical Trial.
-
Maternal Vitamin D Status Correlates to Leukocyte Antigenic Responses in Breastfeeding Infants.Nutrients. 2022 Mar 17;14(6):1266. doi: 10.3390/nu14061266. Nutrients. 2022. PMID: 35334923 Free PMC article. Clinical Trial.
References
-
- Yılmaz B, Aygün C. & Çetinoğlu E. Vitamin D levels in newborns and association with neonatal hypocalcemia. J. Matern. Neonatal Med 31, 1889–1893 (2018). - PubMed
-
- Antonucci R, Locci C, Clemente MG, Chicconi E. & Antonucci L. Vitamin D deficiency in childhood: old lessons and current challenges. J. Pediatr. Endocrinol. Metab 31, 247–260 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

