Paracrine control of α-cell glucagon exocytosis is compromised in human type-2 diabetes
- PMID: 32312960
- PMCID: PMC7171169
- DOI: 10.1038/s41467-020-15717-8
Paracrine control of α-cell glucagon exocytosis is compromised in human type-2 diabetes
Abstract
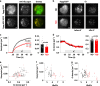
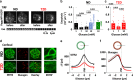
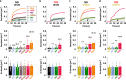
Glucagon is released from pancreatic α-cells to activate pathways that raise blood glucose. Its secretion is regulated by α-cell-intrinsic glucose sensing and paracrine control through insulin and somatostatin. To understand the inadequately high glucagon levels that contribute to hyperglycemia in type-2 diabetes (T2D), we analyzed granule behavior, exocytosis and membrane excitability in α-cells of 68 non-diabetic and 21 T2D human donors. We report that exocytosis is moderately reduced in α-cells of T2D donors, without changes in voltage-dependent ion currents or granule trafficking. Dispersed α-cells have a non-physiological V-shaped dose response to glucose, with maximal exocytosis at hyperglycemia. Within intact islets, hyperglycemia instead inhibits α-cell exocytosis, but not in T2D or when paracrine inhibition by insulin or somatostatin is blocked. Surface expression of somatostatin-receptor-2 is reduced in T2D, suggesting a mechanism for the observed somatostatin resistance. Thus, elevated glucagon in human T2D may reflect α-cell insensitivity to paracrine inhibition at hyperglycemia.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

