Structural basis of astrocytic Ca2+ signals at tripartite synapses
- PMID: 32312988
- PMCID: PMC7170846
- DOI: 10.1038/s41467-020-15648-4
Structural basis of astrocytic Ca2+ signals at tripartite synapses
Erratum in
-
Author Correction: Structural basis of astrocytic Ca2+ signals at tripartite synapses.Nat Commun. 2020 May 18;11(1):2541. doi: 10.1038/s41467-020-16453-9. Nat Commun. 2020. PMID: 32424204 Free PMC article.
Abstract
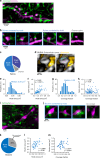
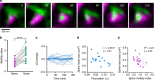
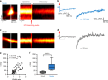
Astrocytic Ca2+ signals can be fast and local, supporting the idea that astrocytes have the ability to regulate single synapses. However, the anatomical basis of such specific signaling remains unclear, owing to difficulties in resolving the spongiform domain of astrocytes where most tripartite synapses are located. Using 3D-STED microscopy in living organotypic brain slices, we imaged the spongiform domain of astrocytes and observed a reticular meshwork of nodes and shafts that often formed loop-like structures. These anatomical features were also observed in acute hippocampal slices and in barrel cortex in vivo. The majority of dendritic spines were contacted by nodes and their sizes were correlated. FRAP experiments and Ca2+ imaging showed that nodes were biochemical compartments and Ca2+ microdomains. Mapping astrocytic Ca2+ signals onto STED images of nodes and dendritic spines showed they were associated with individual synapses. Here, we report on the nanoscale organization of astrocytes, identifying nodes as a functional astrocytic component of tripartite synapses that may enable synapse-specific communication between neurons and astrocytes.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Araque, A., Parpura, V., Sanzgiri, R. P. & Haydon, P. G. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci.22, 208–215 (1999). - PubMed
-
- Witcher, M. R., Kirov, S. A. & Harris, K. M. Plasticity of perisynaptic astroglia during synaptogenesis in the mature rat hippocampus. Glia55, 13–23 (2007). - PubMed
-
- Di Castro, M. A. et al. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci.14, 1276–1284 (2011). - PubMed
-
- Panatier, A. et al. Astrocytes are endogenous regulators of basal transmission at central synapses. Cell146, 785–798 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

