The Nlrp3 inflammasome as a "rising star" in studies of normal and malignant hematopoiesis
- PMID: 32313108
- PMCID: PMC7266743
- DOI: 10.1038/s41375-020-0827-8
The Nlrp3 inflammasome as a "rising star" in studies of normal and malignant hematopoiesis
Abstract
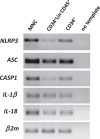
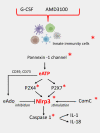
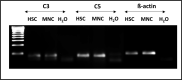
Recent investigations indicate that hematopoiesis is coregulated by innate immunity signals and by pathways characteristic of the activation of innate immunity cells that also operate in normal hematopoietic stem progenitor cells (HSPCs). This should not be surprising because of the common developmental origin of these cells from a hemato/lymphopoietic stem cell. An important integrating factor is the Nlrp3 inflammasome, which has emerged as a major sensor of changes in body microenvironments, cell activation, and cell metabolic activity. It is currently the best-studied member of the inflammasome family expressed in hematopoietic and lymphopoietic cells, including also HSPCs. It is proposed as playing a role in (i) the development and expansion of HSPCs, (ii) their release from bone marrow (BM) into peripheral blood (PB) in stress situations and during pharmacological mobilization, (iii) their homing to BM after transplantation, and (iv) their aging and the regulation of hematopoietic cell metabolism. The Nlrp3 inflammasome is also involved in certain hematological pathologies, including (i) myelodysplastic syndrome, (ii) myeloproliferative neoplasms, (iii) leukemia, and (iv) graft-versus-host disease (GvHD) after transplantation. The aim of this review is to shed more light on this intriguing intracellular protein complex that has become a "rising star" in studies focused on both normal steady-state and pathological hematopoiesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

