Clinical and echocardiographic benefit of Sacubitril/Valsartan in a real-world population with HF with reduced ejection fraction
- PMID: 32313194
- PMCID: PMC7170843
- DOI: 10.1038/s41598-020-63801-2
Clinical and echocardiographic benefit of Sacubitril/Valsartan in a real-world population with HF with reduced ejection fraction
Abstract
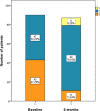
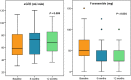
The aim of this study was to evaluate the effects of Sacubitril/Valsartan (S/V) on clinical, laboratory and echocardiographic parameters and outcomes in a real-world population with heart failure with reduced ejection fraction (HFrEF). This was a prospective observational study enrolling patients with HFrEF undergoing treatment with S/V. The primary outcome was the composite of cardiac death and HF rehospitalization at 12 months follow-up; secondary outcomes were all-cause death, cardiac death and the occurrence of rehospitalization for worsening HF. The clinical outcome was compared with a retrospective cohort of 90 HFrEF patients treated with standard medical therapy. The study included 90 patients (66.1 ± 11.7 years) treated with S/V. The adjusted regression analysis showed a significantly lower risk for the primary outcome (HR:0.31; 95%CI, 0.11-0.83; p = 0.019) and for HF rehospitalization (HR:0.27; 95%CI, 0.08-0.94; p = 0.039) in S/V patients as compared to the control group. A significant improvement in NYHA class, left ventricular ejection fraction, left ventricular end systolic volume and systolic pulmonary arterial pressure was observed up to 6 months. S/V did not affect negatively renal function and was associated with a significantly lower dose of furosemide dose prescribed at 6- and 12-month follow-up. In this study, S/V reduced the risk of HF rehospitalization and cardiac death at 1 year in patients with HFrEF. S/V improved NYHA class, echocardiographic parameters and need of furosemide, and preserved renal function.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ponikowski P, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

