Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging
- PMID: 32313216
- PMCID: PMC7416336
- DOI: 10.1038/s41565-020-0642-4
Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging
Abstract
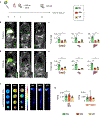
Ischaemic heart disease evokes a complex immune response. However, tools to track the systemic behaviour and dynamics of leukocytes non-invasively in vivo are lacking. Here, we present a multimodal hot-spot imaging approach using an innovative high-density lipoprotein-derived nanotracer with a perfluoro-crown ether payload (19F-HDL) to allow myeloid cell tracking by 19F magnetic resonance imaging. The 19F-HDL nanotracer can additionally be labelled with zirconium-89 and fluorophores to detect myeloid cells by in vivo positron emission tomography imaging and optical modalities, respectively. Using our nanotracer in atherosclerotic mice with myocardial infarction, we observed rapid myeloid cell egress from the spleen and bone marrow by in vivo 19F-HDL magnetic resonance imaging. Concurrently, using ex vivo techniques, we showed that circulating pro-inflammatory myeloid cells accumulated in atherosclerotic plaques and at the myocardial infarct site. Our multimodality imaging approach is a valuable addition to the immunology toolbox, enabling the study of complex myeloid cell behaviour dynamically.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
References for Method section
-
- Nakashima Y, Plump AS, Raines EW, Breslow JL & Ross R ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb 14, 133–40 (1994). - PubMed
-
- Plump AS et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–53 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL125703/HL/NHLBI NIH HHS/United States
- R01 HL128264/HL/NHLBI NIH HHS/United States
- P01 HL131478/HL/NHLBI NIH HHS/United States
- R01 DA049547/DA/NIDA NIH HHS/United States
- R01 EB009638/EB/NIBIB NIH HHS/United States
- R01 CA220234/CA/NCI NIH HHS/United States
- R01 HL144072/HL/NHLBI NIH HHS/United States
- R01 HL117829/HL/NHLBI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- R01 HL135878/HL/NHLBI NIH HHS/United States
- R01 HL143814/HL/NHLBI NIH HHS/United States
- R01 HL096576/HL/NHLBI NIH HHS/United States
- R35 HL139598/HL/NHLBI NIH HHS/United States
- R01 HL118440/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

