Inhaled biguanides and mTOR inhibition for influenza and coronavirus (Review)
- PMID: 32313883
- PMCID: PMC7170270
- DOI: 10.3892/wasj.2020.42
Inhaled biguanides and mTOR inhibition for influenza and coronavirus (Review)
Abstract
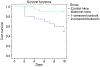
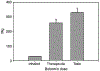
The mammalian target of rapamycin (mTOR) signaling pathway senses and responds to nutrient availability, energy sufficiency, stress, hormones and mitogens to modulate protein synthesis. Rapamycin is a bacterial product that can inhibit mTOR via the PI3K/AKT/mTOR pathway. mTOR signaling is necessary for the development of influenza and modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. In one human study, it was found that the treatment of severe H1N1 influenza‑related pneumonia with rapamycin and steroids improved the outcome. However, in other studies, immunosuppression with systemic steroids, and possibly rapamycin as well, was associated with an increased morbidity/mortality and a prolonged viral replication. In order to avoid the systemic side-effects, some investigators have postulated that the inhalation of rapamycin would be desirable. However, the inhalation of rapamycin, with its well-documented lung toxicity, could be contraindicated. Another class of drug, biguanides, can also inhibit mTOR, but have no lung toxicity. Biguanides are widely used small molecule drugs prescribed as oral anti-diabetics that have exhibited considerable promise in oncology. During the 1971 outbreak of influenza, diabetic patients treated with the biguanides, phenformin and buformin, had a lower incidence of infection than diabetics treated with sulfonylureas or insulin. Both buformin and phenformin reduce the mortality of influenza in mice; phenformin is less effective than buformin. The inhalation of buformin or phenformin for influenza may be an effective novel treatment strategy that would limit the risk of systemic side-effects associated with biguanides due to the low inhaled dose. Coronavirus disease 2019 (COVID-19) is an infectious disease caused by SARS-CoV-2, a virus closely related to the SARS virus. The disease is the cause of the 2019-2020 coronavirus outbreak. It is primarily spread between individuals via small droplets emitted from infected individuals when breathing or coughing. PI3K/AKT/mTOR signaling responses play important roles in MERS-CoV infection and may represent a novel drug target for therapeutic intervention strategies. The present review article discusses the effects of biguanides on influenza and coronavirus.
Keywords: biguanides; buformin; influenza; inhaled; mammalian target of rapamycin.
Conflict of interest statement
Competing interests The authors declare that they have no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
