Structural and functional characterization of the severe fever with thrombocytopenia syndrome virus L protein
- PMID: 32313945
- PMCID: PMC7261188
- DOI: 10.1093/nar/gkaa253
Structural and functional characterization of the severe fever with thrombocytopenia syndrome virus L protein
Erratum in
-
Correction to 'structural and functional characterization of the Severe fever with thrombocytopenia syndrome virus L protein'.Nucleic Acids Res. 2023 Apr 11;51(6):2996-2997. doi: 10.1093/nar/gkad174. Nucleic Acids Res. 2023. PMID: 36881762 Free PMC article. No abstract available.
Abstract
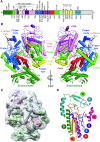
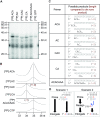
The Bunyavirales order contains several emerging viruses with high epidemic potential, including Severe fever with thrombocytopenia syndrome virus (SFTSV). The lack of medical countermeasures, such as vaccines and antivirals, is a limiting factor for the containment of any virus outbreak. To develop such antivirals a profound understanding of the viral replication process is essential. The L protein of bunyaviruses is a multi-functional and multi-domain protein performing both virus transcription and genome replication and, therefore, is an ideal drug target. We established expression and purification procedures for the full-length L protein of SFTSV. By combining single-particle electron cryo-microscopy and X-ray crystallography, we obtained 3D models covering ∼70% of the SFTSV L protein in the apo-conformation including the polymerase core region, the endonuclease and the cap-binding domain. We compared this first L structure of the Phenuiviridae family to the structures of La Crosse peribunyavirus L protein and influenza orthomyxovirus polymerase. Together with a comprehensive biochemical characterization of the distinct functions of SFTSV L protein, this work provides a solid framework for future structural and functional studies of L protein-RNA interactions and the development of antiviral strategies against this group of emerging human pathogens.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- McMullan L.K., Folk S.M., Kelly A.J., MacNeil A., Goldsmith C.S., Metcalfe M.G., Batten B.C., Albarino C.G., Zaki S.R., Rollin P.E. et al. .. A new phlebovirus associated with severe febrile illness in Missouri. N. Engl. J. Med. 2012; 367:834–841. - PubMed
-
- Maes P., Alkhovsky S.V., Bao Y., Beer M., Birkhead M., Briese T., Buchmeier M.J., Calisher C.H., Charrel R.N., Choi I.R. et al. .. Taxonomy of the family Arenaviridae and the order Bunyavirales: update 2018. Arch. Virol. 2018; 163:2295–2310. - PubMed
-
- Finberg R.W., Lanno R., Anderson D., Fleischhackl R., van Duijnhoven W., Kauffman R.S., Kosoglou T., Vingerhoets J., Leopold L.. Phase 2b study of Pimodivir (JNJ-63623872) as monotherapy or in combination with oseltamivir for treatment of acute uncomplicated seasonal influenza A: TOPAZ trial. J. Infect. Dis. 2019; 219:1026–1034. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

