Formation of a 2D Meta-stable Oxide by Differential Oxidation of AgCu Alloys
- PMID: 32314585
- PMCID: PMC7304822
- DOI: 10.1021/acsami.0c03963
Formation of a 2D Meta-stable Oxide by Differential Oxidation of AgCu Alloys
Abstract
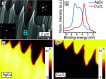
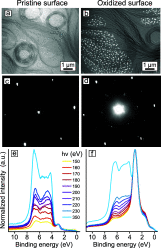
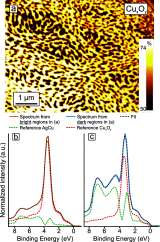
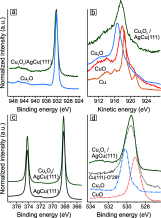
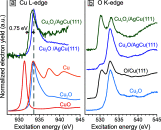
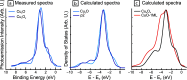
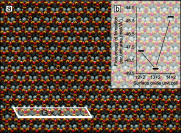
Metal alloy catalysts can develop complex surface structures when exposed to reactive atmospheres. The structures of the resulting surfaces have intricate relationships with a myriad of factors, such as the affinity of the individual alloying elements to the components of the gas atmosphere and the bond strengths of the multitude of low-energy surface compounds that can be formed. Identifying the atomic structure of such surfaces is a prerequisite for establishing structure-property relationships, as well as for modeling such catalysts in ab initio calculations. Here, we show that an alloy, consisting of an oxophilic metal (Cu) diluted into a noble metal (Ag), forms a meta-stable two-dimensional oxide monolayer, when the alloy is subjected to oxidative reaction conditions. The presence of this oxide is correlated with selectivity in the corresponding test reaction of ethylene epoxidation. In the present study, using a combination of in situ, ex situ, and theoretical methods (NAP-XPS, XPEEM, LEED, and DFT), we determine the structure to be a two-dimensional analogue of Cu2O, resembling a single lattice plane of Cu2O. The overlayer holds a pseudo-epitaxial relationship with the underlying noble metal. Spectroscopic evidence shows that the oxide's electronic structure is qualitatively distinct from its three-dimensional counterpart, and because of weak electronic coupling with the underlying noble metal, it exhibits metallic properties. These findings provide precise details of this peculiar structure and valuable insights into how alloying can enhance catalytic properties.
Keywords: 2-dimensional material; XPS; dilute alloy; meta-stable; oxide monolayer.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Reuter K.; Stampf C.; Scheffler M.. Ab Initio Atomistic Thermodynamics and Statistical Mechanics of Surface Properties and Functions. In Handbook of Materials Modeling: Methods; Yip S., Ed.; Springer Netherlands: Dordrecht, 2005; pp 149–194.
-
- Greiner M. T.; Cao J.; Jones T. E.; Beeg S.; Skorupska K.; Carbonio E. A.; Sezen H.; Amati M.; Gregoratti L.; Willinger M.-G.; Knop-Gericke A.; Schlögl R. Phase Coexistence of Multiple Copper Oxides on AgCu Catalysts during Ethylene Epoxidation. ACS Catal. 2018, 8, 2286–2295. 10.1021/acscatal.7b04187. - DOI
-
- Linic S.; Jankowiak J.; Barteau M. A. Selectivity Driven Design of Bimetallic Ethylene Epoxidation Catalysts from First Principles. J. Catal. 2004, 224, 489–493. 10.1016/j.jcat.2004.03.007. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

