Phenotypic plasticity as a mechanism of cave colonization and adaptation
- PMID: 32314737
- PMCID: PMC7173965
- DOI: 10.7554/eLife.51830
Phenotypic plasticity as a mechanism of cave colonization and adaptation
Abstract
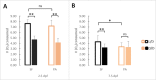
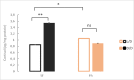
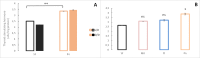
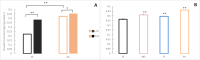
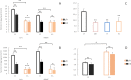
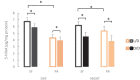
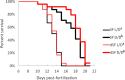
A widely accepted model for the evolution of cave animals posits colonization by surface ancestors followed by the acquisition of adaptations over many generations. However, the speed of cave adaptation in some species suggests mechanisms operating over shorter timescales. To address these mechanisms, we used Astyanax mexicanus, a teleost with ancestral surface morphs (surface fish, SF) and derived cave morphs (cavefish, CF). We exposed SF to completely dark conditions and identified numerous altered traits at both the gene expression and phenotypic levels. Remarkably, most of these alterations mimicked CF phenotypes. Our results indicate that many cave-related traits can appear within a single generation by phenotypic plasticity. In the next generation, plasticity can be further refined. The initial plastic responses are random in adaptive outcome but may determine the subsequent course of evolution. Our study suggests that phenotypic plasticity contributes to the rapid evolution of cave-related traits in A. mexicanus.
Keywords: Astyanax mexicanus; cave adaptation; cavefish; colonization; evolutionary biology; rapid evolution; total darkness.
Plain language summary
The Mexican tetra is a fish that has two forms: a surface-dwelling form, which has eyes and silvery grey appearance, and a cave-dwelling form, which is blind and has lost its pigmentation. Recent studies have shown that the cave-dwelling form evolved rapidly within the last 200,000 years from an ancestor that lived at the surface. The recent evolution of the cave-dwelling form of the tetra poses an interesting evolutionary question: how did the surface-dwelling ancestor of the tetra quickly adapt to the new and challenging environment found in the caves? ‘Phenotypic plasticity’ is a phenomenon through which a single set of genes can produce different observable traits depending on the environment. An example of phenotypic plasticity occurs in response to diet: in animals, poor diets can lead to an increase in the size of the digestive organs and to the animals eating more. To see if surface-dwelling tetras can quickly adapt to cave environments through phenotypic plasticity, Bilandžija et al. have exposed these fish to complete darkness (the major feature of the cave environment) for two years. After spending up to two years in the dark, these fish were compared to normal surface-dwelling and cave-dwelling tetras. Results revealed that surface-dwelling tetras raised in the dark exhibited traits associated with cave-dwelling tetras. These traits included changes in the activity of many genes involved in diverse processes, resistance to starvation, metabolism, and levels of hormones and molecules involved in neural signaling, which could lead to changes in behavior. However, the fish also exhibited traits, including an increase in the cells responsible for pigmentation, that would have no obvious benefit in the darkness. Even though the changes observed require no genetic mutations, they can help or hinder the fish’s survival once they occur, possibly determining subsequent evolution. Thus, a trait beneficial for surviving in the dark that appears simply through phenotypic plasticity may eventually be selected for and genetic mutations that encode it more reliably may appear too. These results shed light on how species may quickly adapt to new environments without accumulating genetic mutations, which can take hundreds of thousands of years. They also may help to explain how colonizer species succeed in challenging environments. The principles described by Bilandžija et al. can be applied to different organisms adapting to new environments, and may help understand the role of phenotypic plasticity in evolution.
© 2020, Bilandžija et al.
Conflict of interest statement
HB, BH, MS, GM, MN, AK, RG, HĆ, MP, KR, WJ No competing interests declared
Figures



References
-
- Andrews S. FastQC: A quality control tool for high throughput sequence data. 2010 http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
-
- Barr TC. Cave ecology and the evolution of troglobites. In: Dobzhansky T, Hecht M. K, Steere W. C, editors. Evolutionary Biology. Springer; 1968. pp. 35–102. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

