Use of Smart Technology for the Early Diagnosis of Complications After Cardiac Surgery: The Box 2.0 Study Protocol
- PMID: 32314974
- PMCID: PMC7201318
- DOI: 10.2196/16326
Use of Smart Technology for the Early Diagnosis of Complications After Cardiac Surgery: The Box 2.0 Study Protocol
Abstract
Background: Atrial fibrillation (AF), sternal wound infection, and cardiac decompensation are complications that can occur after cardiac surgery. Early detection of these complications is clinically relevant, as early treatment is associated with better clinical outcomes. Remote monitoring with the use of a smartphone (mobile health [mHealth]) might improve the early detection of complications after cardiac surgery.
Objective: The primary aim of this study is to compare the detection rate of AF diagnosed with an mHealth solution to the detection rate of AF diagnosed with standard care. Secondary objectives include detection of sternal wound infection and cardiac decompensation, as well as assessment of quality of life, patient satisfaction, and cost-effectiveness.
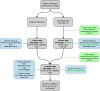
Methods: The Box 2.0 is a study with a prospective intervention group and a historical control group for comparison. Patients undergoing cardiac surgery at Leiden University Medical Center are eligible for enrollment. In this study, 365 historical patients will be used as controls and 365 other participants will be asked to receive either The Box 2.0 intervention consisting of seven home measurement devices along with a video consultation 2 weeks after discharge or standard cardiac care for 3 months. Patient information will be analyzed according to the intention-to-treat principle. The Box 2.0 devices include a blood pressure monitor, thermometer, weight scale, step count watch, single-lead electrocardiogram (ECG) device, 12-lead ECG device, and pulse oximeter.
Results: The study started in November 2018. The primary outcome of this study is the detection rate of AF in both groups. Quality of life is measured with the five-level EuroQol five-dimension (EQ-5D-5L) questionnaire. Cost-effectiveness is calculated from a society perspective using prices from Dutch costing guidelines and quality of life data from the study. In the historical cohort, 93.9% (336/358) completed the EQ-5D-5L and patient satisfaction questionnaires 3 months after cardiac surgery.
Conclusions: The rationale and design of a study to investigate mHealth devices in postoperative cardiac surgery patients are presented. The first results are expected in September 2020.
Trial registration: ClinicalTrials.gov NCT03690492; http://clinicaltrials.gov/show/NCT03690492.
International registered report identifier (irrid): DERR1-10.2196/16326.
Keywords: ambulatory electrocardiography; atrial fibrillation; cardiac surgery; mHealth; postoperative care.
©Tom E Biersteker, Mark J Boogers, Robert AF de Lind van Wijngaarden, Rolf HH Groenwold, Serge A Trines, Anouk P van Alem, Charles JHJ Kirchhof, Nicolette van Hof, Robert JM Klautz, Martin J Schalij, Roderick W Treskes. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 21.04.2020.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT, Investigators of the Ischemia ResearchEducation Foundation. Multicenter Study of Perioperative Ischemia Research Group A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004 Apr 14;291(14):1720–9. doi: 10.1001/jama.291.14.1720. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

