Circular inference in bistable perception
- PMID: 32315404
- PMCID: PMC7405786
- DOI: 10.1167/jov.20.4.12
Circular inference in bistable perception
Abstract
When facing ambiguous images, the brain switches between mutually exclusive interpretations, a phenomenon known as bistable perception. Despite years of research, a consensus on whether bistability is driven primarily by bottom-up or top-down mechanisms has not been achieved. Here, we adopted a Bayesian approach to reconcile these two theories. Fifty-five healthy participants were exposed to an adaptation of the Necker cube paradigm, in which we manipulated sensory evidence and prior knowledge. Manipulations of both sensory evidence and priors significantly affected the way participants perceived the Necker cube. However, we observed an interaction between the effect of the cue and the effect of the instructions, a finding that is incompatible with Bayes-optimal integration. In contrast, the data were well predicted by a circular inference model. In this model, ambiguous sensory evidence is systematically biased in the direction of current expectations, ultimately resulting in a bistable percept.
Figures
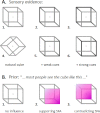


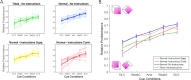


References
-
- Bishop C. (2006). Pattern Recognition and Machine Learning. Berlin: Springer.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

