Isolation, culture, and downstream characterization of primary microglia and astrocytes from adult rodent brain and spinal cord
- PMID: 32315669
- PMCID: PMC7293863
- DOI: 10.1016/j.jneumeth.2020.108742
Isolation, culture, and downstream characterization of primary microglia and astrocytes from adult rodent brain and spinal cord
Abstract
Background: Neuroimmunologists aspire to understand the interactions between neurons, microglia, and astrocytes in the CNS. To study these cells, researchers work with either immortalized cell lines or primary cells acquired from animal tissue. Primary cells reflect in vivo characteristics and functionality compared to immortalized cells; however, they are challenging to acquire and maintain.
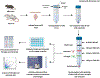
New method: Established protocols to harvest primary glia use neonatal rodents, here we provide a method for simultaneously isolating microglia and astrocytes from brain and/or spinal cord from adult rodents. We utilized a discontinuous percoll density gradient enabling easy discrimination of these cell populations without enzymatic digestion or complex sorting techniques.
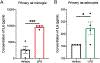
Results: We found cells isolated from the percoll interface between 70 %-50 % were microglia, as they express ionizing calcium-binding adaptor molecule 1 (Iba1) in immunocytochemistry and CD11bhi and CD45lo using flow cytometry. Isolated cells from the 50 %-30 % interface were astrocytes as they express glial fibrillary acidic protein (GFAP) in immunocytochemistry and Glutamate aspartate transporter (GLAST)-1 using flow cytometry. Cultured microglia and astrocytes showed a functional increase in IL-6 production after treatment of lipopolysaccharide (LPS).
Comparison with existing methods: Our method allows for rapid isolation of both microglia and astrocytes in one protocol with relatively few resources, preserves cellular phenotype, and yields high cell numbers without magnetic or antibody sorting.
Conclusion: Here we show a novel, single protocol to isolate microglia and astrocytes from brain and spinal cord tissue, allowing for culturing and other downstream applications from the cells of animals of various ages, which will be useful for researchers investigating these two major glial cell types from the brain or spinal cord of the same rodent.
Keywords: Astrocytes; Culture; ELISA; Flow cytometry; IL-6; Immunocytochemistry; Isolation; Microglia.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have declared no conflict of interest.
Figures

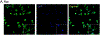


References
-
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia, 2007; 55: 233–8. - PubMed
-
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol, 1990; 27: 229–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

