A Comparative Analysis of Oocyte Development in Mammals
- PMID: 32316494
- PMCID: PMC7226043
- DOI: 10.3390/cells9041002
A Comparative Analysis of Oocyte Development in Mammals
Abstract
Sexual reproduction requires the fertilization of a female gamete after it has undergone optimal development. Various aspects of oocyte development and many molecular actors in this process are shared among mammals, but phylogeny and experimental data reveal species specificities. In this chapter, we will present these common and distinctive features with a focus on three points: the shaping of the oocyte transcriptome from evolutionarily conserved and rapidly evolving genes, the control of folliculogenesis and ovulation rate by oocyte-secreted Growth and Differentiation Factor 9 and Bone Morphogenetic Protein 15, and the importance of lipid metabolism.
Keywords: Bmp15; Gdf9; evolution; gene expression; lipids; mammals; oocyte; posttranscriptional control.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

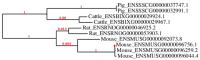
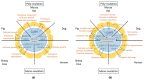

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

