Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase
- PMID: 32316603
- PMCID: PMC7226087
- DOI: 10.3390/biom10040624
Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase
Abstract
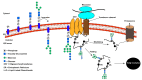
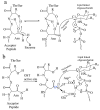
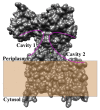
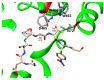
Asparagine-linked glycosylation, also known as N-linked glycosylation is an essential and highly conserved post-translational protein modification that occurs in all three domains of life. This modification is essential for specific molecular recognition, protein folding, sorting in the endoplasmic reticulum, cell-cell communication, and stability. Defects in N-linked glycosylation results in a class of inherited diseases known as congenital disorders of glycosylation (CDG). N-linked glycosylation occurs in the endoplasmic reticulum (ER) lumen by a membrane associated enzyme complex called the oligosaccharyltransferase (OST). In the central step of this reaction, an oligosaccharide group is transferred from a lipid-linked dolichol pyrophosphate donor to the acceptor substrate, the side chain of a specific asparagine residue of a newly synthesized protein. The prokaryotic OST enzyme consists of a single polypeptide chain, also known as single subunit OST or ssOST. In contrast, the eukaryotic OST is a complex of multiple non-identical subunits. In this review, we will discuss the biochemical and structural characterization of the prokaryotic, yeast, and mammalian OST enzymes. This review explains the most recent high-resolution structures of OST determined thus far and the mechanistic implication of N-linked glycosylation throughout all domains of life. It has been shown that the ssOST enzyme, AglB protein of the archaeon Archaeoglobus fulgidus, and the PglB protein of the bacterium Campylobactor lari are structurally and functionally similar to the catalytic Stt3 subunit of the eukaryotic OST enzyme complex. Yeast OST enzyme complex contains a single Stt3 subunit, whereas the human OST complex is formed with either STT3A or STT3B, two paralogues of Stt3. Both human OST complexes, OST-A (with STT3A) and OST-B (containing STT3B), are involved in the N-linked glycosylation of proteins in the ER. The cryo-EM structures of both human OST-A and OST-B complexes were reported recently. An acceptor peptide and a donor substrate (dolichylphosphate) were observed to be bound to the OST-B complex whereas only dolichylphosphate was bound to the OST-A complex suggesting disparate affinities of two OST complexes for the acceptor substrates. However, we still lack an understanding of the independent role of each eukaryotic OST subunit in N-linked glycosylation or in the stabilization of the enzyme complex. Discerning the role of each subunit through structure and function studies will potentially reveal the mechanistic details of N-linked glycosylation in higher organisms. Thus, getting an insight into the requirement of multiple non-identical subunits in the N-linked glycosylation process in eukaryotes poses an important future goal.
Keywords: congenital disorders of glycosylation; cryo-EM structures; human oligosaccharyltransferase; mechanism of N-linked glycosylation; membrane proteins; yeast oligosaccharyltransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

