Multi-Methodological Quantitative Taste Assessment of Anti-Tuberculosis Drugs to Support the Development of Palatable Paediatric Dosage Forms
- PMID: 32316692
- PMCID: PMC7238065
- DOI: 10.3390/pharmaceutics12040369
Multi-Methodological Quantitative Taste Assessment of Anti-Tuberculosis Drugs to Support the Development of Palatable Paediatric Dosage Forms
Abstract
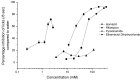
The unpalatability of antituberculosis drugs is often cited as a major cause of non-adherence in children, yet limited quantitative taste assessment data are available. The aim of this research was to quantify the bitterness of isoniazid, rifampicin, pyrazinamide, and ethambutol dihydrochloride using two in vivo (a human taste panel and a rat brief-access taste aversion (BATA) model) and one in vitro (sensor) method. The response of the Insent TS-5000Z electronic tongue was compared to the in vivo drug concentration found to elicit and suppress half the maximum taste response (EC50 in human and IC50 in rats). Using dose-relevant concentrations, an overarching rank order of bitterness was derived (rifampicin > ethambutol > pyrazinamid~isoniazid). In vitro, only ethambutol exhibited a linear response for all sensors/concentrations. Based on the EC50/IC50 generated, a 'taste index' was proposed to allow for anticipation of the likelihood of taste issues in practice, taking in account the saturability in the saliva and therapeutic doses; ethambutol and isoniazid were found to be the worst tasting using this measure. The study presents the first quantitative taste analysis of these life-saving drugs and has allowed for a comparison of three methods of obtaining such data. Such information allows the operator to identify and prioritise the drugs requiring taste masking to produce palatable formulations.
Keywords: BATA model; biomimetic sensors; electronic tongue; human taste panel; palatability; taste assessment; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- World Health Organization . Global Tuberculosis Report 2017. WHO; Geneva, Switzerland: 2017. pp. 1–4.
-
- Hong Kong Chest Service British Medical Research Council Controlled Trial of 2, 4, and 6 Months of Pyrazinamide in 6 Month, Three Times Weekly Regimens for Smear Positive Pulmonary Tuberculosis, Including an Assessment of a Combined Preparation of Isoniazid, Rifampin and Pyrazinamide. [(accessed on 16 April 2020)];Am. Rev. Respir. Dis. 1990 143:700–706. doi: 10.1164/ajrccm/143.4_Pt_1.700. Available online: - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

