Study protocol: a cluster randomized trial to evaluate the effectiveness and implementation of onsite GeneXpert testing at community health centers in Uganda (XPEL-TB)
- PMID: 32316993
- PMCID: PMC7171793
- DOI: 10.1186/s13012-020-00988-y
Study protocol: a cluster randomized trial to evaluate the effectiveness and implementation of onsite GeneXpert testing at community health centers in Uganda (XPEL-TB)
Abstract
Background: Delays in diagnosis and treatment of tuberculosis (TB) remain common in high-burden countries. To improve case detection, substantial investments have been made to scale-up Xpert MTB/RIF (Xpert), a cartridge-based nucleic acid amplification test that can detect TB within 2 hours, as a replacement for sputum smear microscopy. However, the optimal strategy for implementation of Xpert testing remains unclear.
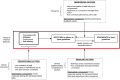
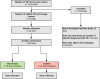
Methods: The Xpert Performance Evaluation for Linkage to Tuberculosis Care (XPEL-TB) trial uses an ultra-pragmatic, hybrid type II effectiveness-implementation design to assess the effectiveness and implementation of a streamlined strategy for delivery of Xpert testing in real-world settings. Twenty health centers with TB microscopy units were selected to participate in the trial, with ten health centers randomized to the intervention strategy (onsite molecular testing using GeneXpert Edge, process redesign to facilitate same-day TB diagnosis and treatment, and performance feedback) or routine care (onsite sputum smear microscopy plus referral of sputum samples to Xpert testing sites). The primary outcome is the number of patients with microbiologically confirmed TB who were initiated on treatment within 14 days of presentation to the health center, which reflects successful completion of the TB diagnostic evaluation process. Secondary outcomes include health outcomes (6-month vital status), as well as measures of the reach, adoption, and implementation of the intervention strategy.
Discussion: The design elements and implementation approach for the XPEL-TB trial were intentionally selected to minimize disruptions to routine care procedures, with the goal of limiting their influence on key primary and secondary outcomes. Trial findings may result in increased support and funding for rapid, onsite molecular testing as the standard-of-care for all patients being evaluated for TB.
Trial registration: US National Institutes of Health's ClinicalTrials.gov, NCT03044158. Registered 06 February 2017. Pan African Clinical Trials Registry, PACTR201610001763265. Registered 03 September 2016.
Keywords: Cluster randomized trial; Effectiveness-implementation design; Pragmatic trial; Tuberculosis; Xpert MTB/RIF.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- World Health Organization. Monitoring of Xpert MTB/RIF roll-out. 2016. Available from: https://www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/. [Accessed 05 Mar 2020].
-
- The Foundation for Innovative New Diagnostics. GeneXpert. Available from: https://www.finddx.org/pricing/genexpert/. [Accessed 05 Mar 2020].