Developing therapeutic monoclonal antibodies at pandemic pace
- PMID: 32317764
- PMCID: PMC7173776
- DOI: 10.1038/s41587-020-0512-5
Developing therapeutic monoclonal antibodies at pandemic pace
Abstract
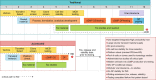
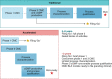
The time from discovery to proof-of-concept trials could be reduced to 5–6 months from a traditional timeline of 10–12 months.
Conflict of interest statement
The author is employed by Vir Biotechnology, which is developing monoclonal antibodies for the treatment of pandemic diseases, such as COVID-19 and influenza.
Figures
Comment in
-
Alternative hosts as the missing link for equitable therapeutic protein production.Nat Biotechnol. 2021 Apr;39(4):404-407. doi: 10.1038/s41587-021-00884-w. Nat Biotechnol. 2021. PMID: 33782611 No abstract available.
References
-
- The Antibody Society. Antibody therapeutics approved or in regulatory review in the EU or US. https://www.antibodysociety.org/resources/approved-antibodies/ (2020).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

