Tussilagone Inhibits Osteoclastogenesis and Periprosthetic Osteolysis by Suppressing the NF-κB and P38 MAPK Signaling Pathways
- PMID: 32317967
- PMCID: PMC7146087
- DOI: 10.3389/fphar.2020.00385
Tussilagone Inhibits Osteoclastogenesis and Periprosthetic Osteolysis by Suppressing the NF-κB and P38 MAPK Signaling Pathways
Abstract
Background: Aseptic prosthetic loosening is one of the main factors causing poor prognosis of limb function after joint replacement and requires troublesome revisional surgery. It is featured by wear particle-induced periprosthetic osteolysis mediated by excessive osteoclasts activated in inflammatory cell context. Some natural compounds show antiosteoclast traits with high cost-efficiency and few side effects. Tussilagone (TUS), which is the main functional extract from Tussilago farfara generally used for relieving cough, asthma, and eliminating phlegm in traditional medicine has been proven to appease several RAW264.7-mediated inflammatory diseases via suppressing osteoclast-related signaling cascades. However, whether and how TUS can improve aseptic prosthetic loosening via modulating osteoclast-mediated bone resorption still needs to be answered.
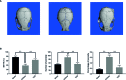
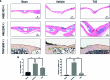
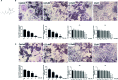
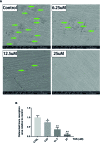
Methods: We established a murine calvarial osteolysis model to detect the preventative effect of TUS on osteolysis in vivo. Micro-CT scanning and histomorphometric analysis were used to determine the variation of bone resorption and osteoclastogenesis. The anti-osteoclast-differentiation and anti-bone-resorption bioactivities of TUS in vitro were investigated using bone slice resorption pit evaluation, and interference caused by cytotoxicity of TUS was excluded according to the CCK-8 assay results. Quantitative polymerase chain reaction (qPCR) analysis was applied to prove the decreased expression of osteoclast-specific genes after TUS treatment. The inhibitory effect of TUS on NF-κB and p38 MAPK signaling pathways was testified by Western blot and NF-κB-linked luciferase reporter gene assay.
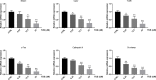
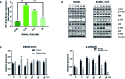
Results: TUS better protected bones against osteolysis in murine calvarial osteolysis model with reduced osteoclasts than those in the control group. In vitro studies also showed that TUS exerted antiosteoclastogenesis and anti-bone-resorption effects in both bone marrow macrophages (BMMs) and RAW264.7 cells, as evidenced by the decline of osteoclast-specific genes according to qPCR. Western blotting revealed that TUS treatment inhibited IκBα degradation and p38 phosphorylation.
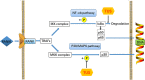
Conclusions: Collectively, our studies proved for the first time that TUS inhibits osteoclastogenesis by suppressing the NF-κB and p38 MAPK signaling pathways, therefore serving as a potential natural compound to treat periprosthetic osteolysis-induced aseptic prosthetic loosening.
Keywords: MAPK; NF-κB; aseptic prosthetic loosening; osteoclast; p38; periprosthetic osteolysis; tussilagone.
Copyright © 2020 Hu, Yin, Chen, Jiang, Yang, Cao, Li, Liu, Peng and Dou.
Figures
Similar articles
-
Cimifugin Suppresses NF-κB Signaling to Prevent Osteoclastogenesis and Periprosthetic Osteolysis.Front Pharmacol. 2021 Sep 29;12:724256. doi: 10.3389/fphar.2021.724256. eCollection 2021. Front Pharmacol. 2021. PMID: 34658863 Free PMC article.
-
Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis.Front Immunol. 2023 Oct 4;14:1274679. doi: 10.3389/fimmu.2023.1274679. eCollection 2023. Front Immunol. 2023. PMID: 37860014 Free PMC article. Review.
-
Scutellarin inhibits RANKL-mediated osteoclastogenesis and titanium particle-induced osteolysis via suppression of NF-κB and MAPK signaling pathway.Int Immunopharmacol. 2016 Nov;40:458-465. doi: 10.1016/j.intimp.2016.09.031. Epub 2016 Oct 8. Int Immunopharmacol. 2016. PMID: 27728897
-
Zingerone attenuates Ti particle-induced inflammatory osteolysis by suppressing the NF-κB signaling pathway in osteoclasts.Int Immunopharmacol. 2023 Feb;115:109720. doi: 10.1016/j.intimp.2023.109720. Epub 2023 Jan 21. Int Immunopharmacol. 2023. PMID: 37724956
-
Involvement of NF-κB/NLRP3 axis in the progression of aseptic loosening of total joint arthroplasties: a review of molecular mechanisms.Naunyn Schmiedebergs Arch Pharmacol. 2022 Jul;395(7):757-767. doi: 10.1007/s00210-022-02232-4. Epub 2022 Apr 4. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35377011 Review.
Cited by
-
Cimifugin Suppresses NF-κB Signaling to Prevent Osteoclastogenesis and Periprosthetic Osteolysis.Front Pharmacol. 2021 Sep 29;12:724256. doi: 10.3389/fphar.2021.724256. eCollection 2021. Front Pharmacol. 2021. PMID: 34658863 Free PMC article.
-
Punicalin Attenuates Breast Cancer-Associated Osteolysis by Inhibiting the NF-κB Signaling Pathway of Osteoclasts.Front Pharmacol. 2021 Nov 12;12:789552. doi: 10.3389/fphar.2021.789552. eCollection 2021. Front Pharmacol. 2021. PMID: 34867423 Free PMC article.
-
Mechanism of regulating macrophages/osteoclasts in attenuating wear particle-induced aseptic osteolysis.Front Immunol. 2023 Oct 4;14:1274679. doi: 10.3389/fimmu.2023.1274679. eCollection 2023. Front Immunol. 2023. PMID: 37860014 Free PMC article. Review.
-
Review on effects and mechanisms of plant-derived natural products against breast cancer bone metastasis.Heliyon. 2024 Sep 13;10(18):e37894. doi: 10.1016/j.heliyon.2024.e37894. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39318810 Free PMC article. Review.
-
Tussilagone protects acute lung injury from PM2.5 via alleviating Hif-1α/NF-κB-mediated inflammatory response.Environ Toxicol. 2022 May;37(5):1198-1210. doi: 10.1002/tox.23476. Epub 2022 Feb 3. Environ Toxicol. 2022. PMID: 35112795 Free PMC article.
References
LinkOut - more resources
Full Text Sources

