Novel Human Polymorphisms Define a Key Role for the SLC26A6-STAS Domain in Protection From Ca2+-Oxalate Lithogenesis
- PMID: 32317970
- PMCID: PMC7154107
- DOI: 10.3389/fphar.2020.00405
Novel Human Polymorphisms Define a Key Role for the SLC26A6-STAS Domain in Protection From Ca2+-Oxalate Lithogenesis
Abstract
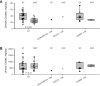
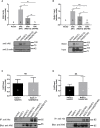
Impaired homeostasis of the carboxylic acids oxalate and citrate, dramatically increases the risk for the formation of Ca2+-oxalate kidney stones, which is the most common form of kidney stones in humans. Renal homeostasis of oxalate and citrate is controlled by complex mechanisms including epithelial transport proteins such as the oxalate transporter, SLC26A6, and the citrate transporters, the SLC13's. These transporters interact via the SLC26A6-STAS domain in vitro, however, the role of the Sulfate Transporter and Anti-Sigma factor antagonist (STAS) domain in Ca2+-oxalate stone formation was not investigated in humans. Here, we report two novel human SLC26A6 polymorphisms identified in the STAS domain of SLC26A6 in two heterozygous carriers. Intriguingly, these individuals have low urinary citrate, but different clinical manifestations. Our in vitro experiments indicate that the homolog mutations of SLC26A6(D23H/D673N) and SLC26A6(D673N) alone abolished the expression and function of SLC26A6, and impaired the regulation of SLC13-mediated citrate transport by SLC26A6. On the other hand, the SLC26A6(R621G) variant showed reduced SLC26A6 protein expression and membrane trafficking, retained full transport activity, but impaired the regulation of the citrate transporter. Accordingly, the human SLC26A6(D23H/D673N) carrier showed a dramatic reduction in urinary citrate concentrations which resulted in Ca2+-oxalate stones formation, as opposed to the carrier of SLC26A6(R621G). Our findings indicate that the human SLC26A6-STAS domain mutations differentially impair SLC26A6 expression, function, and regulation of citrate transporters. This interferes with citrate and oxalate homeostasis thus potentially predisposes to Ca2+-oxalate kidney stones.
Keywords: NaDC-1; SLC26A6; citrate; kidney stones; oxalate.
Copyright © 2020 Shimshilashvili, Aharon, Moe and Ohana.
Figures


References
-
- Aronson P. S. (2010). Role of SLC26A6-mediated Cl(-)-oxalate exchange in renal physiology and pathophysiology. J. Nephrol. 23 Suppl 16, S158–S164. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

