Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract
- PMID: 32318067
- PMCID: PMC7147503
- DOI: 10.3389/fimmu.2020.00575
Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract
Abstract
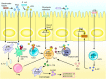
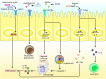
The intestinal tract is the largest digestive organ in the human body. It is colonized by, and consistently exposed to, a myriad of microorganisms, including bifidobacteria, lactobacillus, Escherichia coli, enterococcus, clostridium perfringens, and pseudomonas. To protect the body from potential pathogens, the intestinal tract has evolved regional immune characteristics. These characteristics are defined by its unique structure, function, and microenvironment, which differ drastically from those of the common central and peripheral immune organs. The intestinal microenvironment created by the intestinal flora and its products significantly affects the immune function of the region. In turn, specific diseases regulate and influence the composition of the intestinal flora. A constant interplay occurs between the intestinal flora and immune system. Further, the intestinal microenvironment can be reconstructed by probiotic use or microbiota transplantation, functioning to recalibrate the immune homeostasis, while also contributing to the treatment or amelioration of diseases. In this review, we summarize the relationship between the intestinal flora and the occurrence and development of diseases as an in-turn effect on intestinal immunity. We also discuss improved immune function as it relates to non-specific and specific immunity. Further, we discuss the proliferation, differentiation and secretion of immune cells, within the intestinal region following remodeling of the microenvironment as a means to ameliorate and treat diseases. Finally, we suggest strategies for improved utilization of intestinal flora.
Keywords: fecal microbiota transplantation; intestinal flora; intestinal tract; probiotic; regional immune system.
Copyright © 2020 Zhou, Yuan, Zhang, Guo, Li, Li, Xiong and Zeng.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

