New Insights in the Pathogenesis and Therapy of Cold Agglutinin-Mediated Autoimmune Hemolytic Anemia
- PMID: 32318071
- PMCID: PMC7154122
- DOI: 10.3389/fimmu.2020.00590
New Insights in the Pathogenesis and Therapy of Cold Agglutinin-Mediated Autoimmune Hemolytic Anemia
Abstract
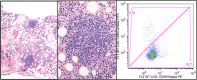
Autoimmune hemolytic anemias mediated by cold agglutinins can be divided into cold agglutinin disease (CAD), which is a well-defined clinicopathologic entity and a clonal lymphoproliferative disorder, and secondary cold agglutinin syndrome (CAS), in which a similar picture of cold-hemolytic anemia occurs secondary to another distinct clinical disease. Thus, the pathogenesis in CAD is quite different from that of polyclonal autoimmune diseases such as warm-antibody AIHA. In both CAD and CAS, hemolysis is mediated by the classical complement pathway and therefore can result in generation of anaphylotoxins, such as complement split product 3a (C3a) and, to some extent, C5a. On the other hand, infection and inflammation can act as triggers and drivers of hemolysis, exemplified by exacerbation of CAD in situations with acute phase reaction and the role of specific infections (particularly Mycoplasma pneumoniae and Epstein-Barr virus) as causes of CAS. In this review, the putative mechanisms behind these phenomena will be explained along with other recent achievements in the understanding of pathogenesis in these disorders. Therapeutic approaches have been directed against the clonal lymphoproliferation in CAD or the underlying disease in CAS. Currently, novel targeted treatments, in particular complement-directed therapies, are also being rapidly developed and will be reviewed.
Keywords: autoimmune hemolytic anemia; cold agglutinin disease; complement; inflammation; lymphoproliferative; therapy.
Copyright © 2020 Berentsen.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

