In vitro Implementation of Photopolymerizable Hydrogels as a Potential Treatment of Intracranial Aneurysms
- PMID: 32318555
- PMCID: PMC7146053
- DOI: 10.3389/fbioe.2020.00261
In vitro Implementation of Photopolymerizable Hydrogels as a Potential Treatment of Intracranial Aneurysms
Abstract
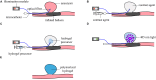
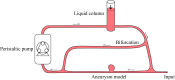
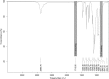
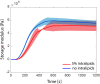
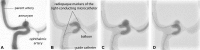
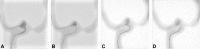
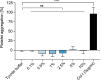
Intracranial aneurysms are increasingly being treated with endovascular therapy, namely coil embolization. Despite being minimally invasive, partial occlusion and recurrence are more frequent compared to open surgical clipping. Therefore, an alternative treatment is needed, ideally combining minimal invasiveness and long-term efficiency. Herein, we propose such an alternative treatment based on an injectable, radiopaque and photopolymerizable polyethylene glycol dimethacrylate hydrogel. The rheological measurements demonstrated a viscosity of 4.86 ± 1.70 mPa.s, which was significantly lower than contrast agent currently used in endovascular treatment (p = 0.42), allowing the hydrogel to be injected through 430 μm inner diameter microcatheters. Photorheology revealed fast hydrogel solidification in 8 min due to the use of a new visible photoinitiator. The addition of an iodinated contrast agent in the precursor contributed to the visibility of the precursor injection under fluoroscopy. Using a customized light-conducting microcatheter and illumination module, the hydrogel was implanted in an in vitro silicone aneurysm model. Specifically, in situ fast and controllable injection and photopolymerization of the developed hydrogel is shown to be feasible in this work. Finally, the precursor and the polymerized hydrogel exhibit no toxicity for the endothelial cells. Photopolymerizable hydrogels are expected to be promising candidates for future intracranial aneurysm treatments.
Keywords: in situ photopolymerization; injectable hydrogels; intracranial aneurysms (IA); light-conducting microcatheter; polyethylene glycol dimethacrylate.
Copyright © 2020 Poupart, Schmocker, Conti, Moser, Nuss, Grützmacher, Mosimann and Pioletti.
Figures





References
-
- Abdihalim M., Watanabe M., Chaudhry S. A., Jagadeesan B., Suri M. F. K., Qureshi A. I. (2014). Are coil compaction and aneurysmal growth two distinct etiologies leading to recurrence following endovascular treatment of intracranial aneurysm? J. Neuroimaging 24 171–175. 10.1111/j.1552-6569.2012.00786.x - DOI - PubMed
-
- Bonneville F., Sourour N., Biondi A. (2006). Intracranial aneurysms: an overview. Neuroimaging Clin. N. Am. 16 371–382. - PubMed
LinkOut - more resources
Full Text Sources

