Imaging the Cell Morphological Response to 3D Topography and Curvature in Engineered Intestinal Tissues
- PMID: 32318564
- PMCID: PMC7154059
- DOI: 10.3389/fbioe.2020.00294
Imaging the Cell Morphological Response to 3D Topography and Curvature in Engineered Intestinal Tissues
Abstract
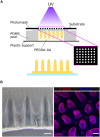
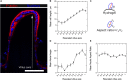
While conventional cell culture methodologies have relied on flat, two-dimensional cell monolayers, three-dimensional engineered tissues are becoming increasingly popular. Often, engineered tissues can mimic the complex architecture of native tissues, leading to advancements in reproducing physiological functional properties. In particular, engineered intestinal tissues often use hydrogels to mimic villi structures. These finger-like protrusions of a few hundred microns in height have a well-defined topography and curvature. Here, we examined the cell morphological response to these villus-like microstructures at single-cell resolution using a novel embedding method that allows for the histological processing of these delicate hydrogel structures. We demonstrated that by using photopolymerisable poly(ethylene) glycol as an embedding medium, the villus-like microstructures were successfully preserved after sectioning with vibratome or cryotome. Moreover, high-resolution imaging of these sections revealed that cell morphology, nuclei orientation, and the expression of epithelial polarization markers were spatially encoded along the vertical axis of the villus-like microstructures and that this cell morphological response was dramatically affected by the substrate curvature. These findings, which are in good agreement with the data reported for in vivo experiments on the native tissue, are likely to be the origin of more physiologically relevant barrier properties of engineered intestinal tissues when compared with standard monolayer cultures. By showcasing this example, we anticipate that the novel histological embedding procedure will have a positive impact on the study of epithelial cell behavior on three-dimensional substrates in both physiological and pathological situations.
Keywords: cell morphology; cell orientation; confocal microscopy; histological section; hydrogel scaffold; small intestine; substrate curvature; villus.
Copyright © 2020 Altay, Tosi, García-Díaz and Martínez.
Figures