ADAM12 silencing promotes cellular apoptosis by activating autophagy in choriocarcinoma cells
- PMID: 32319603
- PMCID: PMC7115740
- DOI: 10.3892/ijo.2020.5007
ADAM12 silencing promotes cellular apoptosis by activating autophagy in choriocarcinoma cells
Abstract
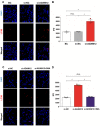
ADAM metallopeptidase domain 12 (ADAM12) has been demonstrated to mediate cell proliferation and apoptosis resistance in several types of cancer cells. However, the effect of ADAM12 silencing on the proliferation and apoptosis of choriocarcinoma cells remains unknown. The present study revealed that ADAM12 silencing significantly inhibited cellular activity and proliferation in the human choriocarcinoma JEG3 cell line and increased the rate of apoptosis. In addition, ADAM12 silencing significantly increased the expression levels of the autophagy proteins microtubule‑associated protein‑light‑chain 3 (LC3B) and autophagy related 5 (ATG5) and the fluorescence density of LC3B in JEG‑3 cells. However, the suppression of autophagy by 3‑methyladenine could block ADAM12 silencing‑induced cellular apoptosis. ADAM12 silencing reduced the levels of the inflammatory factors interleukin‑1β, interferon‑γ and TNF‑α, and inactivated nuclear p65‑NF‑κB and p‑mTOR in JEG‑3 cells. The downregulation of p‑mTOR expression by ADAM12 silencing was rescued in 3‑methyladenine‑treated JEG‑3 cells, indicating that mTOR might participate in the autophagy‑mediated pro‑apoptotic effect of ADAM12 silencing. In conclusion, ADAM12 silencing promoted cellular apoptosis in human choriocarcinoma JEG3 cells, which might be associated with autophagy and the mTOR response. These findings indicate that ADAM12 silencing might be a potential novel therapeutic target for choriocarcinoma.
Keywords: ADAM12; choriocarcinoma cell; proliferation; apoptosis; autophagy.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
