HDGF enhances VEGF‑dependent angiogenesis and FGF‑2 is a VEGF‑independent angiogenic factor in non‑small cell lung cancer
- PMID: 32319650
- PMCID: PMC7251661
- DOI: 10.3892/or.2020.7580
HDGF enhances VEGF‑dependent angiogenesis and FGF‑2 is a VEGF‑independent angiogenic factor in non‑small cell lung cancer
Abstract
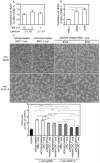
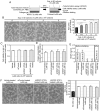
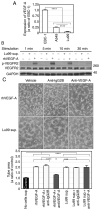
Non‑small cell lung cancer (NSCLC) accounts for over 80% of all diagnosed lung cancer cases. Lung cancer is the leading cause of cancer‑related deaths worldwide. Most NSCLC cells overexpress vascular endothelial growth factor‑A (VEGF‑A) which plays a pivotal role in tumour angiogenesis. Anti‑angiogenic therapies including VEGF‑A neutralisation have significantly improved the response rates, progression‑free survival and overall survival of patients with NSCLC. However, the median survival of these patients is shorter than 18 months, suggesting that NSCLC cells secrete VEGF‑independent angiogenic factors, which remain unknown. We aimed to explore these factors in human NSCLC cell lines, A549, Lu99 and EBC‑1 using serum‑free culture, to which only EBC‑1 cells could adapt. By mass spectrometry, we identified 1,007 proteins in the culture supernatant derived from EBC‑1 cells. Among the identified proteins, interleukin‑8 (IL‑8), macrophage migration inhibitory factor (MIF), galectin‑1, midkine (MK), IL‑18, galectin‑3, VEGF‑A, hepatoma‑derived growth factor (HDGF), osteopontin (OPN), connective tissue growth factor (CTGF) and granulin (GRN) are known to be involved in angiogenesis. Tube formation, neutralisation and RNA interference assays revealed that VEGF‑A and HDGF function as angiogenic factors in EBC‑1 cells. To confirm whether VEGF‑A and HDGF also regulate angiogenesis in the other NSCLC cell lines, we established a novel culture method. NSCLC cells were embedded in collagen gel and cultured three‑dimensionally. Tube formation, neutralisation and RNA interference assays using the three‑dimensional (3D) culture supernatant showed that VEGF‑A and HDGF were not angiogenic factors in Lu99 cells. By gene microarray in EBC‑1 and Lu99 cells, we identified 61 mRNAs expressed only in Lu99 cells. Among these mRNAs, brain‑derived neurotrophic factor (BDNF), fibroblast growth factor‑2 (FGF‑2) and FGF‑5 are known to be involved in angiogenesis. Tube formation and neutralisation assays clarified that FGF‑2 functions as an angiogenic factor in Lu99 cells. These results indicate that HDGF enhances VEGF‑dependent angiogenesis and that FGF‑2 is a VEGF‑independent angiogenic factor in human NSCLC cells.
Keywords: angiogenesis; fibroblast growth factor-2; hepatoma-derived growth factor; non-small cell lung cancer; vascular endothelial growth factor.
Figures







References
-
- Inoue M, Sawada N, Matsuda T, Iwasaki M, Sasazuki S, Shimazu T, Shibuya K, Tsugane S. Attributable causes of cancer in Japan in 2005-systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol. 2012;23:1362–1369. doi: 10.1093/annonc/mdr437. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

