Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials
- PMID: 32320027
- PMCID: PMC7177662
- DOI: 10.1001/jamadermatol.2020.0465
Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients With Facial Acne: Two Phase 3 Randomized Clinical Trials
Abstract
Importance: Acne is a common, multifactorial skin condition, and treatments with novel mechanisms have been elusive.
Objective: To assess the safety and efficacy of clascoterone cream, 1%, a novel topical androgen receptor inhibitor, in 2 phase 3 randomized clinical trials (CB-03-01/25 and CB-03-01/26).
Design, setting, and participants: Two identical, multicenter, randomized, vehicle-controlled, double-blind, phase 3 studies conducted from November 2015 to April 2018 evaluated the efficacy and safety of use of clascoterone cream, 1%, in males and nonpregnant females 9 years and older with moderate or severe facial acne as scored on the Investigator's Global Assessment scale. Participants were enrolled if they had 30 to 75 inflammatory lesions and 30 to 100 noninflammatory lesions.
Interventions: Patients were randomized to treatment with clascoterone cream, 1%, or vehicle cream and applied approximately 1 g to the whole face twice daily for 12 weeks.
Main outcomes and measures: Treatment success was defined as an Investigator's Global Assessment score of 0 (clear) or 1 (almost clear), and a 2-grade or greater improvement from baseline and absolute change from baseline in noninflammatory and inflammatory lesion counts at week 12. Safety measures included adverse event frequency and severity.
Results: A total of 1440 patients were randomzied in 2 studies. In CB-03-01/25, 353 participants were randomized to treatment with clascoterone cream, 1% (median [range] age, 18.0 [10-58] years; 221 [62.6%] female), and 355 participants were randomized to treatment with vehicle cream (median [range] age, 18.0 [9-50] years; 215 (60.6%) female); in CB-03-01/26, 369 participants were randomized to treatment with clascoterone cream, 1% (median [range] age, 18.0 [10-50] years; 243 [65.9%] female), and 363 participants were randomized to treatment with vehicle cream (median [range] age, 18.0 [range, 11-42] years; 221 [60.9%] female). At week 12, treatment success rates in CB-03-01/25 and CB-03-01/26 with clascoterone cream, 1%, were 18.4% (point estimate, 2.3; 95% CI, 1.4-3.8; P < .001) and 20.3% (point estimate, 3.7; 95% CI, 2.2-6.3; P < .001) vs 9.0% and 6.5% with vehicle, respectively. At week 12, in both CB-03-01/25 and CB-03-01/26, treatment with clascoterone cream, 1%, resulted in a significant reduction in absolute noninflammatory lesions from baseline to -19.4 (point estimate difference, -6.4; 95% CI, -10.3 to -2.6; P < .001) and -19.4 (point estimate difference, -8.6; 95% CI, -12.3 to -4.9; P < .001) vs -13.0 and -10.8 with vehicle, respectively, as well as a reduction in inflammatory lesions from baseline to -19.3 (point estimate difference, -3.8; 95% CI, -6.4 to -1.3; P < .001) and -20.0 (point estimate difference, -7.4; 95% CI, -9.8 to -5.1; P < .001) vs -15.5 and -12.6 with vehicle, respectively. Adverse events rates were low and mostly mild; the predominant local skin reaction was trace or mild erythema.
Conclusions and relevance: Use of clascoterone cream, 1%, for acne treatment appears to demonstrate favorable efficacy and safety with low adverse event rates.
Trial registration: ClinicalTrials.gov Identifiers: NCT02608450 and NCT02608476.
Conflict of interest statement
Figures
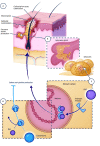

Comment in
-
A New Class of Topical Acne Treatment Addressing the Hormonal Pathogenesis of Acne.JAMA Dermatol. 2020 Jun 1;156(6):619-620. doi: 10.1001/jamadermatol.2020.0464. JAMA Dermatol. 2020. PMID: 32320045 No abstract available.
-
Cortexolone 17α-propionate for hidradenitis suppurativa.Dermatol Ther. 2020 Nov;33(6):e14142. doi: 10.1111/dth.14142. Epub 2020 Sep 4. Dermatol Ther. 2020. PMID: 32761708 No abstract available.
-
Potential Role for Topical Antiandrogens in the Management of Acne Among Patients Receiving Masculinizing Hormone Therapy.JAMA Dermatol. 2020 Dec 1;156(12):1380-1381. doi: 10.1001/jamadermatol.2020.4380. JAMA Dermatol. 2020. PMID: 33146681 No abstract available.
-
Potential Role for Topical Antiandrogens in the Management of Acne Among Patients Receiving Masculinizing Hormone Therapy-Reply.JAMA Dermatol. 2020 Dec 1;156(12):1381. doi: 10.1001/jamadermatol.2020.3341. JAMA Dermatol. 2020. PMID: 33146696 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

