Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction
- PMID: 32320056
- PMCID: PMC7175780
- DOI: 10.1002/14651858.CD013280.pub2
Indocyanine green angiography for preventing postoperative mastectomy skin flap necrosis in immediate breast reconstruction
Abstract
Background: Breast cancer will affect one in eight women during their lifetime. The opportunity to restore the removed tissue and cosmetic appearance is provided by reconstructive breast surgery following skin-sparing mastectomy (SSM). Mastectomy skin flap necrosis (MSFN) is a common complication following SSM breast reconstruction. This postoperative complication can be prevented by intraoperative assessment of mastectomy skin flap viability and intervention when tissue perfusion is compromised. Indocyanine green fluorescence angiography is presumed to be a better predictor of MSFN compared to clinical evaluation alone.
Objectives: To assess the effects of indocyanine green fluorescence angiography (ICGA) for preventing mastectomy skin flap necrosis in women undergoing immediate breast reconstruction following skin-sparing mastectomy. To summarise the different ICGA protocols available for assessment of mastectomy skin flap perfusion in women undergoing immediate breast reconstructions following skin-sparing mastectomy.
Search methods: We searched the Cochrane Breast Cancer Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3, 2019), MEDLINE, Embase, the World Health Organization's International Clinical Trials Registry Platform (ICTRP) and Clinicaltrials.gov in April 2019. In addition, we searched reference lists of published studies.
Selection criteria: We included studies that compared the use of ICGA to clinical evaluation to assess mastectomy skin vascularisation and recruited women undergoing immediate autologous or prosthetic reconstructive surgery following SSM for confirmed breast malignancy or high risk of developing breast cancer.
Data collection and analysis: Two review authors independently assessed the risk of bias of the included nonrandomised studies and extracted data on postoperative outcomes, including postoperative MSFN, reoperation, autologous flap necrosis, dehiscence, infection, haematoma and seroma, and patient-related outcomes. The quality of the evidence was assessed using the GRADE approach and we constructed two 'Summary of finding's tables: one for the comparison of ICGA to clinical evaluation on a per patient basis and one on a per breast basis.
Main results: Nine nonrandomised cohort studies met the inclusion criteria and involved a total of 1589 women with 2199 breast reconstructions. We included seven retrospective and two prospective cohort studies. Six studies reported the number of MSFN on a per breast basis for a total of 1435 breasts and three studies reported the number of MSFN on a per patient basis for a total of 573 women. Five studies reported the number of other complications on a per breast basis for a total of 1370 breasts and four studies reported the number on a per patient basis for a total of 613 patients. Therefore, we decided to pool data separately. Risk of bias for each included nonrandomised study was assessed using the Newcastle-Ottawa Scale for cohort studies. There was serious concern with risk of bias due to the nonrandomised study design of all included studies and the low comparability of cohorts in most studies. The quality of the evidence was found to be very low, after downgrading the quality of evidence twice for imprecision based on the small sample sizes and low number of events in the included studies. Postoperative complications on a per patient basis We are uncertain about the effect of ICGA on MSFN (RR 0.79, 95% CI 0.40 to 1.56; three studies, 573 participants: very low quality of evidence), infection rates (RR 0.91, 95% CI 0.60 to 1.40; four studies, 613 participants: very low quality of evidence), haematoma rates (RR 0.87, 95% CI 0.30 to 2.53; two studies, 459 participants: very low quality of evidence) and seroma rates (RR 1.68, 95% CI 0.41 to 6.80; two studies, 408 participants: very low quality of evidence) compared to the clinical group. We found evidence that ICGA may reduce reoperation rates (RR 0.50, 95% CI 0.35 to 0.72; four studies, 613 participants: very low quality of evidence). One study considered dehiscence as an outcome. In this single study, dehiscence was observed in 2.2% of participants (4/184) in the ICGA group compared to 0.5% of participants (1/184) in the clinical group (P = 0.372). The RR was 4.00 (95% CI 0.45 to 35.45; one study; 368 participants; very low quality of evidence). Postoperative complications on a per breast basis We found evidence that ICGA may reduce MSFN (RR 0.62, 95% CI 0.48 to 0.82; six studies, 1435 breasts: very low quality of evidence), may reduce reoperation rates (RR 0.65, 95% CI 0.47 to 0.92; five studies, 1370 breasts: very low quality of evidence) and may reduce infection rates (RR 0.65, 95% CI 0.44 to 0.97; five studies, 1370 breasts: very low quality of evidence) compared to the clinical group. We are uncertain about the effect of ICGA on haematoma rates (RR 1.53, CI 95% 0.47 to 4.95; four studies, 1042 breasts: very low quality of evidence) and seroma rates (RR 0.71, 95% CI 0.37 to 1.35; two studies, 528 breasts: very low quality of evidence). None of the studies reported patient-related outcomes. ICGA protocols: eight studies used the SPY System and one study used the Photodynamic Eye imaging system (PDE) to assess MSFN. ICGA protocols in the included studies were not extensively described in most studies.
Authors' conclusions: Although mastectomy skin flap perfusion is performed more frequently using ICGA as a helpful tool, there is a lack of high-quality evidence in the context of randomised controlled trials. The quality of evidence in this review is very low, since only nonrandomised cohort studies have been included. With the results from this review, no conclusions can be drawn about what method of assessment is best to use during breast reconstructive surgery. High-quality randomised controlled studies that compare the use of ICGA to assess MSFN compared to clinical evaluation are needed.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
TP: None known. RS: None known. SK: None known. RH: None known. SQ: None known.
Figures
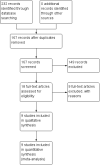
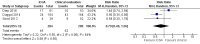











Update of
References
References to studies included in this review
Alstrup 2018 {published data only}
-
- Alstrup T, Christensen BO, Damsgaard TE. ICG angiography in immediate and delayed autologous breast reconstructions: peroperative evaluation and postoperative outcomes. Journal of Plastic Surgery and Hand Surgery 2018;52(5):307‐11. - PubMed
Diep 2016 {published data only}
-
- Diep GK, Hui JY, Marmor S, Cunningham BL, Choudry U, Prtschy PR, et al. Postmastectomy reconstruction outcomes after intraoperative evaluation with indocyanine green angiography versus clinical assessment. Annals of Surgical Oncology 2016;23(12):4080‐5. - PubMed
Duggal 2014 {published data only}
-
- Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthetic Surgery Journal 2014;34(1):61‐5. - PubMed
Gorai 2017 {published data only}
Hammer‐Hansen 2017 {published data only}
-
- Hammer‐Hansen N, Juhl AA, Damsgaard TE. Laser‐assisted indocyanine green angiography in implant‐based immediate breast reconstruction: a retrospective study. Journal of Plastic Surgery and Hand Surgery 2018;52(3):158‐62. - PubMed
Harless 2016 {published data only}
-
- Harless CA, Jacobson SR. Tailoring through technology: a retrospective review of a single surgeon's experience with implant‐based breast reconstruction before and after implementation of laser‐assisted indocyanine green angiography. Breast Journal 2016;22(3):274‐81. - PubMed
Mirhaidari 2018 {published data only}
Rinker 2016 {published data only}
-
- Rinker B. A comparison of methods to assess mastectomy flap viability in skin‐sparing mastectomy and immediate reconstruction: a prospective cohort study. Plastic and Reconstructive Surgery 2016;137(2):395‐401. - PubMed
References to studies excluded from this review
Diep 2019 {published data only}
-
- Diep GK, Marmor S, Kizy S, Huang JL, Jensen EH, Portschy P, et al. The use of indocyanine green angiography in postmastectomy reconstructions: do outcomes improve over time?. Journal of Plastic, Reconstructive & Aesthetic Surgery 2019;72(4):548‐54. - PubMed
Griffiths 2016 {published data only}
Komorowska‐Timek 2010 {published data only}
-
- Komorowska‐Timek E, Gurtner GC. Intraoperative perfusion mapping with laser‐assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plastic and Reconstructive Surgery 2010;125(4):1065‐73. - PubMed
Mattison 2016 {published data only}
-
- Mattison GL, Lewis PG, Gupta SC, Kim HY. SPY imaging use in postmastectomy breast reconstruction patients: preventative or overly conservative?. Plastic and Reconstructive Surgery 2016;138(1):15e‐21e. - PubMed
Munabi 2014 {published data only}
-
- Munabi NCO, Olorunnipa OB, Goltsman D, Rohde CH, Ascherman JA. The ability of intra‐operative perfusion mapping with laser‐assisted indocyanine green angiography to predict mastectomy flap necrosis in breast reconstruction: a prospective trial. Journal of Plastic, Reconstructive & Aesthetic Surgery 2014;67(4):449‐55. - PubMed
Newman 2010 {published data only}
-
- Newman M, Samson M, Tamburrino JF, Swartz KA. Intraoperative laser‐assisted indocyanine green angiography for the evaluation of mastectomy flaps in immediate breast reconstruction. Journal of Reconstructive Microsurgery 2010;26(7):487‐92. - PubMed
Phillips 2012 {published data only}
-
- Phillips BT, Lanier ST, Conkling N, Wang ED, Dagum AB, Ganz JC, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plastic and Reconstructive Surgery 2012;129(8):778‐88e. - PubMed
Wang 2018 {published data only}
-
- Wang CY, Wang CH, Tzeng YS, Lin CT, Chou CY, Chiang IH, et al. Intraoperative assessment of relationship between nipple circulation and incision site in nipple‐sparing mastectomy with implant breast reconstruction using the SPY imaging system. Annals of Plastic Surgery 2018;80(2S Suppl 1):S59‐65. - PubMed
Additional references
Borenstein 2010
-
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods 2010;1:97‐111. - PubMed
Bray 2018
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 26 cancers in 185 countries. CA: Cancer Journal for Clinicians 2018;68:394‐424. - PubMed
Burnier 2017
-
- Burnier P, Niddam J, Bosc R, Hersant B, Meningaud JP. Indocyanine green applications in plastic surgery: a review of the literature. Journal of Plastic, Reconstructive & Aesthetic Surgery 2017;70(6):814‐27. - PubMed
Cornelissen 2018
Denford 2011
-
- Denford S, Harcourt D, Rubin L, Pusic A. Understanding normality: a qualitative analysis of breast cancer patients concepts of normality after mastectomy and reconstructive surgery. Psycho‐oncology 2011;20(5):553‐8. - PubMed
Desmettre 2000
-
- Desmettre T, Devoisselle JM, Mordon S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Survey of Ophthalmology 2000;45(1):15‐27. - PubMed
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. - PubMed
Flower 1973
-
- Flower RW, Hochheimer BF. A clinical technique and apparatus for simultaneous angiography of the separate retinal and choroidal circulations. Investigative Opthalmology 1973;12(4):248‐61. - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc). GRADEpro GDT. Hamilton: McMaster University (developed by Evidence Prime, Inc), 2015.
Hansen 2018
-
- Hansen N, Espino S, Blough JT, Vu MM, Fine NA, Kim JYS. Evaluating mastectomy skin flap necrosis in the extended breast reconstruction risk assessment score for 1‐year prediction of prosthetic reconstruction outcomes. Journal of the American College of Surgeons 2018;227(1):96‐104. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
IKNL 2012
-
- Comprehensive Cancer Centre the Netherlands (IKNL). Netherlands Cancer Registry. www.oncoline.nl/borstkanker (accessed 28 February 2019).
Jeevan 2014
-
- Jeevan R, Cromwell DA, Browne JP, Caddy CM, Pereira J, Sheppard C, et al. Findings of a national comparative audit of mastectomy and breast reconstruction surgery in England. Journal of Plastic, Reconstructive & Aesthetic Surgery 2014;67(10):1333‐44. - PubMed
Jeon 2018
Li 2018
-
- Li K, Zhang Z, Nicoli F, D'Ambrosia C, Xi W, Lazzeri D, et al. Application of indocyanine green in flap surgery: a systematic review. Journal of Reconstructive Microsurgery 2018;34(2):77‐86. - PubMed
Liu 2019
Lohman 2015
-
- Lohman RF, Ozturk CN, Ozturk C, Jayaprakash V, Djohan R. An analysis of current techniques used for intraoperative flap evaluation. Annals of Plastic Surgery 2015;75(6):679‐85. - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. - PubMed
Moyer 2012
-
- Moyer HR, Losken A. Predicting mastectomy skin flap necrosis with indocyanine green angiography: the gray area defined. Plastic and Reconstructive Surgery 2012;129(5):1043‐8. - PubMed
Panchal 2017
Pruimboom 2019
-
- Pruimboom T, Kuijk SMJ, Qiu SS, Bos J, Wieringa FP, Hulst RRWJ, et al. Optimizing indocyanine green fluorescence angiography in reconstructive flap surgery: a systematic review and ex vivo experiment. Surgical Innovations 2020;27(1):103‐19. - PubMed
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 2017
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. 2011. Available from handbook.cochrane.org.
Senkus 2015
-
- Senkus E, Kyriakides S, Ohno S, Penault‐Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2015;26(Suppl 5):v8‐30. - PubMed
Smit 2018
-
- Smit JM, Negenborn VL, Jansen SM, Jaspers MEH, Vries R, Heymans MW, et al. Intraoperative evaluation of perfusion in free flap surgery: a systematic review and meta‐analysis. Microsurgery 2018;38(7):804‐18. - PubMed
Tondu 2018
-
- Tondu T, Tjalma WAA, Thiessen FEF. Breast reconstruction after mastectomy. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2018;230:228‐32. - PubMed
Wells 2008
-
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed prior to 26 March 2020).
Wood 1962
-
- Wood EH. Diagnostic applications of indicator‐dilution technics in congenital heart disease. Circulation Research 1962;10:531‐68. - PubMed
Yeoh 2013
-
- Yeoh MS, Kim DD, Ghali GE. Fluorescence angiography in the assessment of flap perfusion and vitality. Oral and Maxillofacial Surgery Clinics of North America 2013;25(1):61‐6. - PubMed
Zenn 2011
-
- Zenn MR. Fluorescent angiography. Clinics in Plastic Surgery 2011;38(2):293‐300. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

