Rh-Catalyzed Aziridine Ring Expansions to Dehydropiperazines
- PMID: 32320259
- PMCID: PMC8025198
- DOI: 10.1021/acs.orglett.0c01124
Rh-Catalyzed Aziridine Ring Expansions to Dehydropiperazines
Retraction in
-
Retraction of "Rh-Catalyzed Aziridine Ring Expansions to Dehydropiperazines".Org Lett. 2022 Feb 25;24(7):1573. doi: 10.1021/acs.orglett.2c00395. Epub 2022 Feb 11. Org Lett. 2022. PMID: 35147435 Free PMC article. No abstract available.
Abstract
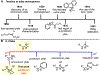
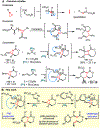
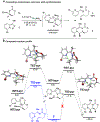
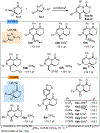
Piperazines are prevalent in pharmaceuticals and natural products, but traditional methods do not typically introduce stereochemical complexity into the ring. To expand access to these scaffolds, we report Rh-catalyzed ring expansions of aziridines and N-sulfonyl-1,2,3-triazoles to furnish dehydropiperazines with excellent diastereocontrol. Productive ring expansion proceeds via a pseudo-1,4-sigmatropic rearrangement of an aziridinium ylide species. However, the structural features of the carbene precursor are important, as pyridotriazoles undergo competing cheletropic extrusion to furnish ketimines.
Figures

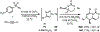
References
-
- Li A-H; Dai L-X; Aggarwal VK Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement. Chem. Rev 1997, 97, 2341–2372. - PubMed
-
- Michaelis A; Gimborn HV Ueber Das Betain Und Cholin Dea Triphenylphosphins. Berichte der deutschen chemischen Gesellschaft. 1894, 27, 272–277.
-
- Wittig VG; Geissler G Zur Reaktionsweise Des Pentaphenyl-Phosphors Und Einiger Derivate. Justus Liebigs Ann. Chem 1953, 580, 44–57.
-
- Staudinger H Ursprung Und Entwicklung in Der Chemie Der Phosphin-Alkylene. Angew. Chem. Int. Ed 1956, 68, 505–532.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

