Microneedles in Smart Drug Delivery
- PMID: 32320365
- PMCID: PMC7906867
- DOI: 10.1089/wound.2019.1122
Microneedles in Smart Drug Delivery
Abstract
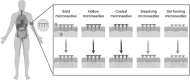
Significance: In biomedical setup, at large, and drug delivery, in particular, transdermal patches, hypodermal needles, and/or dermatological creams with the topical appliance are among the most widely practiced routes for transdermal drug delivery. Owing to the stratum corneum layer of the skin, traditional drug delivery methods are inefficient, and the effect of the administered therapeutic cues is limited. Recent Advances: The current advancement at the microlevel and nanolevel has revolutionized the drug delivery sector. Particularly, various types of microneedles (MNs) are becoming popular for drug delivery applications because of safety, patient compliance, and smart action. Critical Issues: Herein, we reviewed state-of-the-art MNs as a smart and sophisticated drug delivery approach. Following a brief introduction, the drug delivery mechanism of MNs is discussed. Different types of MNs, that is, solid, hollow, coated, dissolving, and hydrogel forming, are discussed with suitable examples. The latter half of the work is focused on the applied perspective and clinical translation of MNs. Furthermore, a detailed overview of clinical applications and future perspectives is also included in this review. Future Directions: Regardless of ongoing technological and clinical advancement, the focus should be diverted to enhance the efficacy and strength of MNs. Besides, the possible immune response or interference should also be avoided for successful clinical translation of MNs as an efficient drug delivery system.
Keywords: drug delivery system; fabrication strategies; influencing factors; microneedles; microneedles types.
Conflict of interest statement
No competing financial interests exist. The content of this article was expressly written by the author(s) listed. No ghostwriters were used to write this article.
Figures







References
-
- Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery–a review. Pharm Sci Technol Today 2000;3:138–145 - PubMed
-
- Raza A, Shen N, Li J, Chen Y, Wang J-Y. Formulation of zein based compression coated floating tablets for enhanced gastric retention and tunable drug release. Eur J Pharm Sci 2019;132:163–173 - PubMed
-
- Tanner T, Marks R. Delivering drugs by the transdermal route: review and comment. Skin Res Technol 2008;14:249–260 - PubMed
-
- Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev 2004;56:581–587 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
