The evaluating prescription opioid changes in veterans (EPOCH) study: Design, survey response, and baseline characteristics
- PMID: 32320421
- PMCID: PMC7176145
- DOI: 10.1371/journal.pone.0230751
The evaluating prescription opioid changes in veterans (EPOCH) study: Design, survey response, and baseline characteristics
Abstract
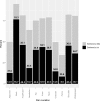
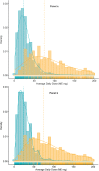
In the United States (US), long-term opioid therapy has been commonly prescribed for chronic pain. Since recognition of the opioid overdose epidemic, clinical practice guidelines have recommended tapering long-term opioids to reduced doses or discontinuation. The Effects of Prescription Opioid Changes for veterans (EPOCH) study is a national population-based prospective observational study of US Veterans Health Administration primary care patients designed to assess effects of evolving opioid prescribing practice on patients treated with long-term opioids for chronic pain. A stratified random sampling design was used to identify a survey sample from the target population of patients treated with opioid analgesics for ≥ 6 months. Demographic, diagnostic, visit, and pharmacy dispensing data were extracted from existing datasets. A 2016 mixed-mode mail and telephone survey collected patient-reported data, including the main patient-reported outcomes of pain-related function (Brief Pain Inventory interference; BPI-I scores 0-10, higher scores = worse) and health-related quality of life. Data on survey participants and non-participants were analyzed to assess potential nonresponse bias. Weights were used to account for design. Linear regression models were used to assess cross-sectional associations of opioid treatment with patient-reported measures. Of 14,160 patients contacted, 9253 (65.4%) completed the survey. Participants were older than non-participants (63.9 ± 10.6 vs. 59.6 ± 13.0 years). The mean number of bothersome pain locations was 6.8 (SE 0.04). Effectiveness of pain treatment and quality of pain care were rated fair or poor by 56.1% and 45.3%, respectively. The opioid daily dosage range was 1.6 to 1038.2 mg, with mean = 50.6 mg (SE 1.1) and median = 30.9 mg (IQR 40.7). Among the 73.2% of patients who did not receive long-acting opioids, the mean daily dosage was 30.4 mg (SE 0.6) and mean BPI-I was 6.4 (SE 00.4). Among patients who received long-acting opioids, the mean daily dosage was 106.2 mg (SE 2.8) and mean BPI-I was 6.8 (SE 0.07). Higher daily dosage was associated with worse pain-related function and quality of life among patients without long-acting opioids, but not among patients with long-acting opioids. Future analyses will use follow-up data to examine effects of opioid dose reduction and discontinuation on patient outcomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

