Targeting Angiotensin-Converting Enzyme-2/Angiotensin-(1-7)/Mas Receptor Axis in the Vascular Progenitor Cells for Cardiovascular Diseases
- PMID: 32321734
- PMCID: PMC7725063
- DOI: 10.1124/mol.119.117580
Targeting Angiotensin-Converting Enzyme-2/Angiotensin-(1-7)/Mas Receptor Axis in the Vascular Progenitor Cells for Cardiovascular Diseases
Abstract
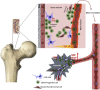
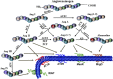
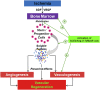
Bone marrow-derived hematopoietic stem/progenitor cells are vasculogenic and play an important role in endothelial health and vascular homeostasis by participating in postnatal vasculogenesis. Progenitor cells are mobilized from bone marrow niches in response to remote ischemic injury and migrate to the areas of damage and stimulate revascularization largely by paracrine activation of angiogenic functions in the peri-ischemic vasculature. This innate vasoprotective mechanism is impaired in certain chronic clinical conditions, which leads to the development of cardiovascular complications. Members of the renin-angiotensin system-angiotensin-converting enzymes (ACEs) ACE and ACE2, angiotensin II (Ang II), Ang-(1-7), and receptors AT1 and Mas-are expressed in vasculogenic progenitor cells derived from humans and rodents. Ang-(1-7), generated by ACE2, is known to produce cardiovascular protective effects by acting on Mas receptor and is considered as a counter-regulatory mechanism to the detrimental effects of Ang II. Evidence has now been accumulating in support of the activation of the ACE2/Ang-(1-7)/Mas receptor pathway by pharmacologic or molecular maneuvers, which stimulates mobilization of progenitor cells from bone marrow, migration to areas of vascular damage, and revascularization of ischemic areas in pathologic conditions. This minireview summarizes recent studies that have enhanced our understanding of the physiology and pharmacology of vasoprotective axis in bone marrow-derived progenitor cells in health and disease. SIGNIFICANCE STATEMENT: Hematopoietic stem progenitor cells (HSPCs) stimulate revascularization of ischemic areas. However, the reparative potential is diminished in certain chronic clinical conditions, leading to the development of cardiovascular diseases. ACE2 and Mas receptor are key members of the alternative axis of the renin-angiotensin system and are expressed in HSPCs. Accumulating evidence points to activation of ACE2 or Mas receptor as a promising approach for restoring the reparative potential, thereby preventing the development of ischemic vascular diseases.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Aicher A, Heeschen C, Dimmeler S. (2004) The role of NOS3 in stem cell mobilization. Trends Mol Med 10:421–425. - PubMed
-
- Altarche-Xifró W, Curato C, Kaschina E, Grzesiak A, Slavic S, Dong J, Kappert K, Steckelings M, Imboden H, Unger T, et al. (2009) Cardiac c-kit+AT2+ cell population is increased in response to ischemic injury and supports cardiomyocyte performance. Stem Cells 27:2488–2497. - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85:221–228. - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967. - PubMed
-
- Awad O, Dedkov EI, Jiao C, Bloomer S, Tomanek RJ, Schatteman GC. (2006) Differential Healing Activities of CD34+ and CD14+ Endothelial Cell Progenitors. Arterioscler Thromb Vasc Biol 26:758–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

