Acute Stress Enhances Associative Learning via Dopamine Signaling in the Ventral Lateral Striatum
- PMID: 32321745
- PMCID: PMC7252483
- DOI: 10.1523/JNEUROSCI.3003-19.2020
Acute Stress Enhances Associative Learning via Dopamine Signaling in the Ventral Lateral Striatum
Abstract
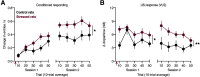
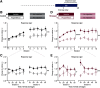
Acute stress transiently increases vigilance, enhancing the detection of salient stimuli in one's environment. This increased perceptual sensitivity is thought to promote the association of rewarding outcomes with relevant cues. The mesolimbic dopamine system is critical for learning cue-reward associations. Dopamine levels in the ventral striatum are elevated following exposure to stress. Together, this suggests that the mesolimbic dopamine system could mediate the influence of acute stress on cue-reward learning. To address this possibility, we examined how a single stressful experience influenced learning in an appetitive pavlovian conditioning task. Male rats underwent an episode of restraint prior to the first conditioning session. This acute stress treatment augmented conditioned responding in subsequent sessions. Voltammetry recordings of mesolimbic dopamine levels demonstrated that acute stress selectively increased reward-evoked dopamine release in the ventral lateral striatum (VLS), but not in the ventral medial striatum. Antagonizing dopamine receptors in the VLS blocked the stress-induced enhancement of conditioned responding. Collectively, these findings illustrate that stress engages dopamine signaling in the VLS to facilitate appetitive learning.SIGNIFICANCE STATEMENT Acute stress influences learning about aversive and rewarding outcomes. Dopamine neurons are sensitive to stress and critical for reward learning. However, it is unclear whether stress regulates reward learning via dopamine signaling. Using fast-scan cyclic voltammetry as rats underwent pavlovian conditioning, we demonstrate that a single stressful experience increases reward-evoked dopamine release in the ventral lateral striatum. This enhanced dopamine signal accompanies a long-lasting increase in conditioned behavioral responding. These findings highlight that the ventral lateral striatum is a node for mediating the effect of stress on reward processing.
Keywords: dopamine; learning; stress; ventral striatum.
Copyright © 2020 the authors.
Figures





Similar articles
-
Critical periods when dopamine controls behavioral responding during Pavlovian learning.Psychopharmacology (Berl). 2022 Sep;239(9):2985-2996. doi: 10.1007/s00213-022-06182-w. Epub 2022 Jul 7. Psychopharmacology (Berl). 2022. PMID: 35796814 Free PMC article.
-
Effects of an acute therapeutic or rewarding dose of amphetamine on acquisition of Pavlovian autoshaping and ventral striatal dopamine signaling.Behav Brain Res. 2018 Jan 15;336:191-203. doi: 10.1016/j.bbr.2017.09.003. Epub 2017 Sep 5. Behav Brain Res. 2018. PMID: 28882695
-
Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats.Horm Behav. 2020 Nov;126:104855. doi: 10.1016/j.yhbeh.2020.104855. Epub 2020 Oct 1. Horm Behav. 2020. PMID: 32991888 Free PMC article.
-
Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems.Ann N Y Acad Sci. 1999 Jun 29;877:412-38. doi: 10.1111/j.1749-6632.1999.tb09280.x. Ann N Y Acad Sci. 1999. PMID: 10415662 Review.
-
Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior.Prog Brain Res. 2000;126:193-215. doi: 10.1016/S0079-6123(00)26015-9. Prog Brain Res. 2000. PMID: 11105648 Review.
Cited by
-
Relationship between stressful life events, coping styles, and schizophrenia relapse.Int J Ment Health Nurs. 2021 Oct;30(5):1149-1159. doi: 10.1111/inm.12865. Epub 2021 May 6. Int J Ment Health Nurs. 2021. PMID: 33960095 Free PMC article.
-
The Granular Retrosplenial Cortex Is Necessary in Male Rats for Object-Location Associative Learning and Memory, But Not Spatial Working Memory or Visual Discrimination and Reversal, in the Touchscreen Operant Chamber.eNeuro. 2024 Jun 18;11(6):ENEURO.0120-24.2024. doi: 10.1523/ENEURO.0120-24.2024. Print 2024 Jun. eNeuro. 2024. PMID: 38844347 Free PMC article.
-
Stress to inflammation and anhedonia: Mechanistic insights from preclinical and clinical models.Neurosci Biobehav Rev. 2023 Sep;152:105307. doi: 10.1016/j.neubiorev.2023.105307. Epub 2023 Jul 6. Neurosci Biobehav Rev. 2023. PMID: 37419230 Free PMC article. Review.
-
Dopamine release and its control over early Pavlovian learning differs between the NAc core and medial NAc shell.Neuropsychopharmacology. 2021 Sep;46(10):1780-1787. doi: 10.1038/s41386-020-00941-z. Epub 2021 Jan 15. Neuropsychopharmacology. 2021. PMID: 33452431 Free PMC article.
-
Mesolimbic dopamine release conveys causal associations.Science. 2022 Dec 23;378(6626):eabq6740. doi: 10.1126/science.abq6740. Epub 2022 Dec 23. Science. 2022. PMID: 36480599 Free PMC article.
References
-
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ (2012) Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci 32:15779–15790. 10.1523/JNEUROSCI.3557-12.2012 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
