Molecular Mechanisms of Non-ionotropic NMDA Receptor Signaling in Dendritic Spine Shrinkage
- PMID: 32321746
- PMCID: PMC7204083
- DOI: 10.1523/JNEUROSCI.0046-20.2020
Molecular Mechanisms of Non-ionotropic NMDA Receptor Signaling in Dendritic Spine Shrinkage
Abstract
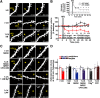
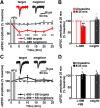
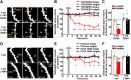
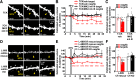
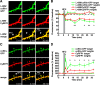
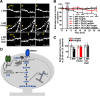
Structural plasticity of dendritic spines is a key component of the refinement of synaptic connections during learning. Recent studies highlight a novel role for the NMDA receptor (NMDAR), independent of ion flow, in driving spine shrinkage and LTD. Yet little is known about the molecular mechanisms that link conformational changes in the NMDAR to changes in spine size and synaptic strength. Here, using two-photon glutamate uncaging to induce plasticity at individual dendritic spines on hippocampal CA1 neurons from mice and rats of both sexes, we demonstrate that p38 MAPK is generally required downstream of non-ionotropic NMDAR signaling to drive both spine shrinkage and LTD. In a series of pharmacological and molecular genetic experiments, we identify key components of the non-ionotropic NMDAR signaling pathway driving dendritic spine shrinkage, including the interaction between NOS1AP (nitric oxide synthase 1 adaptor protein) and neuronal nitric oxide synthase (nNOS), nNOS enzymatic activity, activation of MK2 (MAPK-activated protein kinase 2) and cofilin, and signaling through CaMKII. Our results represent a large step forward in delineating the molecular mechanisms of non-ionotropic NMDAR signaling that can drive shrinkage and elimination of dendritic spines during synaptic plasticity.SIGNIFICANCE STATEMENT Signaling through the NMDA receptor (NMDAR) is vitally important for the synaptic plasticity that underlies learning. Recent studies highlight a novel role for the NMDAR, independent of ion flow, in driving synaptic weakening and dendritic spine shrinkage during synaptic plasticity. Here, we delineate several key components of the molecular pathway that links conformational signaling through the NMDAR to dendritic spine shrinkage during synaptic plasticity.
Keywords: NMDA receptor; dendritic spine; long-term depression; nitric oxide; structural plasticity; two-photon imaging.
Copyright © 2020 the authors.
Figures

Similar articles
-
Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage.J Neurosci. 2015 Sep 2;35(35):12303-8. doi: 10.1523/JNEUROSCI.4289-14.2015. J Neurosci. 2015. PMID: 26338340 Free PMC article.
-
Non-ionotropic NMDA receptor signaling gates bidirectional structural plasticity of dendritic spines.Cell Rep. 2021 Jan 26;34(4):108664. doi: 10.1016/j.celrep.2020.108664. Cell Rep. 2021. PMID: 33503425 Free PMC article.
-
Amyloid-β Causes NMDA Receptor Dysfunction and Dendritic Spine Loss through mGluR1 and AKAP150-Anchored Calcineurin Signaling.J Neurosci. 2024 Sep 11;44(37):e0675242024. doi: 10.1523/JNEUROSCI.0675-24.2024. J Neurosci. 2024. PMID: 39134419 Free PMC article.
-
Behind the scenes: Are latent memories supported by calcium independent plasticity?Hippocampus. 2022 Feb;32(2):73-88. doi: 10.1002/hipo.23332. Epub 2021 Apr 27. Hippocampus. 2022. PMID: 33905147 Free PMC article. Review.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
-
Superoxide and Non-ionotropic Signaling in Neuronal Excitotoxicity.Front Neurosci. 2020 Sep 3;4:861. doi: 10.3389/fnins.2020.00861. eCollection 2020. Front Neurosci. 2020. PMID: 33013314 Free PMC article. Review.
-
L-type Ca2+ channel activation of STIM1-Orai1 signaling remodels the dendritic spine ER to maintain long-term structural plasticity.Proc Natl Acad Sci U S A. 2024 Aug 27;121(35):e2407324121. doi: 10.1073/pnas.2407324121. Epub 2024 Aug 23. Proc Natl Acad Sci U S A. 2024. PMID: 39178228 Free PMC article.
-
Piezo1 ion channels are capable of conformational signaling.Neuron. 2024 Sep 25;112(18):3161-3175.e5. doi: 10.1016/j.neuron.2024.06.024. Epub 2024 Jul 22. Neuron. 2024. PMID: 39043183
-
Ion flux-independent NMDA receptor signaling.Neuropharmacology. 2022 Jun 1;210:109019. doi: 10.1016/j.neuropharm.2022.109019. Epub 2022 Mar 9. Neuropharmacology. 2022. PMID: 35278420 Free PMC article. Review.
-
Reduced d-serine levels drive enhanced non-ionotropic NMDA receptor signaling and destabilization of dendritic spines in a mouse model for studying schizophrenia.Neurobiol Dis. 2022 Aug;170:105772. doi: 10.1016/j.nbd.2022.105772. Epub 2022 May 20. Neurobiol Dis. 2022. PMID: 35605760 Free PMC article.
References
-
- Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, Coyle JT (2013) Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A 110:E2400–E2409. 10.1073/pnas.1304308110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
