Fly eyes are not still: a motion illusion in Drosophila flight supports parallel visual processing
- PMID: 32321749
- PMCID: PMC7272343
- DOI: 10.1242/jeb.212316
Fly eyes are not still: a motion illusion in Drosophila flight supports parallel visual processing
Abstract
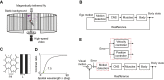
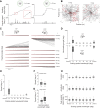
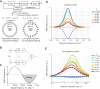
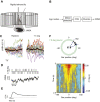
Most animals shift gaze by a 'fixate and saccade' strategy, where the fixation phase stabilizes background motion. A logical prerequisite for robust detection and tracking of moving foreground objects, therefore, is to suppress the perception of background motion. In a virtual reality magnetic tether system enabling free yaw movement, Drosophila implemented a fixate and saccade strategy in the presence of a static panorama. When the spatial wavelength of a vertical grating was below the Nyquist wavelength of the compound eyes, flies drifted continuously and gaze could not be maintained at a single location. Because the drift occurs from a motionless stimulus - thus any perceived motion stimuli are generated by the fly itself - it is illusory, driven by perceptual aliasing. Notably, the drift speed was significantly faster than under a uniform panorama, suggesting perceptual enhancement as a result of aliasing. Under the same visual conditions in a rigid-tether paradigm, wing steering responses to the unresolvable static panorama were not distinguishable from those to a resolvable static pattern, suggesting visual aliasing is induced by ego motion. We hypothesized that obstructing the control of gaze fixation also disrupts detection and tracking of objects. Using the illusory motion stimulus, we show that magnetically tethered Drosophila track objects robustly in flight even when gaze is not fixated as flies continuously drift. Taken together, our study provides further support for parallel visual motion processing and reveals the critical influence of body motion on visuomotor processing. Motion illusions can reveal important shared principles of information processing across taxa.
Keywords: Control; Feedback; Motion vision; Saccade; Stability.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
Divergent visual ecology of Drosophila species drives object-tracking strategies matched to landscape sparsity.Curr Biol. 2024 Oct 21;34(20):4743-4755.e3. doi: 10.1016/j.cub.2024.08.036. Epub 2024 Sep 17. Curr Biol. 2024. PMID: 39293439
-
Drosophila Spatiotemporally Integrates Visual Signals to Control Saccades.Curr Biol. 2017 Oct 9;27(19):2901-2914.e2. doi: 10.1016/j.cub.2017.08.035. Epub 2017 Sep 21. Curr Biol. 2017. PMID: 28943085 Free PMC article.
-
Saccades to remembered targets: the effects of smooth pursuit and illusory stimulus motion.J Neurophysiol. 1996 Dec;76(6):3617-32. doi: 10.1152/jn.1996.76.6.3617. J Neurophysiol. 1996. PMID: 8985862 Clinical Trial.
-
Hybrid visual control in fly flight: insights into gaze shift via saccades.Curr Opin Insect Sci. 2020 Dec;42:23-31. doi: 10.1016/j.cois.2020.08.009. Epub 2020 Sep 5. Curr Opin Insect Sci. 2020. PMID: 32896628 Review.
-
Illusions in action: consequences of inconsistent processing of spatial attributes.Exp Brain Res. 2002 Nov;147(2):135-44. doi: 10.1007/s00221-002-1185-7. Epub 2002 Sep 28. Exp Brain Res. 2002. PMID: 12410328 Review.
Cited by
-
Flies adaptively control flight to compensate for added inertia.Proc Biol Sci. 2023 Oct 11;290(2008):20231115. doi: 10.1098/rspb.2023.1115. Epub 2023 Oct 11. Proc Biol Sci. 2023. PMID: 37817597 Free PMC article.
-
Nested mechanosensory feedback actively damps visually guided head movements in Drosophila.Elife. 2022 Oct 19;11:e80880. doi: 10.7554/eLife.80880. Elife. 2022. PMID: 36259536 Free PMC article.
-
The influence of saccades on yaw gaze stabilization in fly flight.PLoS Comput Biol. 2023 Dec 21;19(12):e1011746. doi: 10.1371/journal.pcbi.1011746. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38127819 Free PMC article.
-
Moving in an Uncertain World: Robust and Adaptive Control of Locomotion from Organisms to Machine Intelligence.Integr Comp Biol. 2024 Nov 21;64(5):1390-1407. doi: 10.1093/icb/icae121. Integr Comp Biol. 2024. PMID: 39090982 Free PMC article. Review.
-
Illusional Perspective across Humans and Bees.Vision (Basel). 2022 May 31;6(2):28. doi: 10.3390/vision6020028. Vision (Basel). 2022. PMID: 35737416 Free PMC article. Review.
References
-
- Aptekar J. W., Keles M. F., Mongeau J.-M., Lu P. M., Frye M. A. and Shoemaker P. A. (2014). Method and software for using m-sequences to characterize parallel components of higher-order visual tracking behavior in Drosophila. Front. Neural Circuits 8, 130 10.3389/fncir.2014.00130 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

