α2,3 linkage of sialic acid to a GPI anchor and an unpredicted GPI attachment site in human prion protein
- PMID: 32321762
- PMCID: PMC7261787
- DOI: 10.1074/jbc.RA120.013444
α2,3 linkage of sialic acid to a GPI anchor and an unpredicted GPI attachment site in human prion protein
Abstract
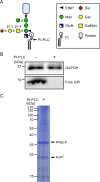
Prion diseases are transmissible, lethal neurodegenerative disorders caused by accumulation of the aggregated scrapie form of the prion protein (PrPSc) after conversion of the cellular prion protein (PrPC). The glycosylphosphatidylinositol (GPI) anchor of PrPC is involved in prion disease pathogenesis, and especially sialic acid in a GPI side chain reportedly affects PrPC conversion. Thus, it is important to define the location and structure of the GPI anchor in human PrPC Moreover, the sialic acid linkage type in the GPI side chain has not been determined for any GPI-anchored protein. Here we report GPI glycan structures of human PrPC isolated from human brains and from brains of a knock-in mouse model in which the mouse prion protein (Prnp) gene was replaced with the human PRNP gene. LC-electrospray ionization-MS analysis of human PrPC from both biological sources indicated that Gly229 is the ω site in PrPC to which GPI is attached. Gly229 in human PrPC does not correspond to Ser231, the previously reported ω site of Syrian hamster PrPC We found that ∼41% and 28% of GPI anchors in human PrPCs from human and knock-in mouse brains, respectively, have N-acetylneuraminic acid in the side chain. Using a sialic acid linkage-specific alkylamidation method to discriminate α2,3 linkage from α2,6 linkage, we found that N-acetylneuraminic acid in PrPC's GPI side chain is linked to galactose through an α2,3 linkage. In summary, we report the GPI glycan structure of human PrPC, including the ω-site amino acid for GPI attachment and the sialic acid linkage type.
Keywords: Creutzfeldt–Jakob disease; MS; glycosylation; glycosylphosphatidylinositol (GPI); neurodegeneration; prion; scrapie; sialic acid.
© 2020 Kobayashi et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Neurodegeneration induced by clustering of sialylated glycosylphosphatidylinositols of prion proteins.J Biol Chem. 2012 Mar 9;287(11):7935-44. doi: 10.1074/jbc.M111.275743. Epub 2012 Jan 19. J Biol Chem. 2012. PMID: 22262833 Free PMC article.
-
Sialic Acid within the Glycosylphosphatidylinositol Anchor Targets the Cellular Prion Protein to Synapses.J Biol Chem. 2016 Aug 12;291(33):17093-101. doi: 10.1074/jbc.M116.731117. Epub 2016 Jun 20. J Biol Chem. 2016. PMID: 27325697 Free PMC article.
-
Sialic Acid on the Glycosylphosphatidylinositol Anchor Regulates PrP-mediated Cell Signaling and Prion Formation.J Biol Chem. 2016 Jan 1;291(1):160-70. doi: 10.1074/jbc.M115.672394. Epub 2015 Nov 9. J Biol Chem. 2016. PMID: 26553874 Free PMC article.
-
The role of GPI-anchored PrP C in mediating the neurotoxic effect of scrapie prions in neurons.Curr Issues Mol Biol. 2010;12(2):119-27. Epub 2009 Sep 18. Curr Issues Mol Biol. 2010. PMID: 19767655 Review.
-
Cell Biology of Prion Protein.Prog Mol Biol Transl Sci. 2017;150:57-82. doi: 10.1016/bs.pmbts.2017.06.018. Epub 2017 Jul 29. Prog Mol Biol Transl Sci. 2017. PMID: 28838675 Review.
Cited by
-
The molecular determinants of a universal prion acceptor.PLoS Pathog. 2024 Sep 10;20(9):e1012538. doi: 10.1371/journal.ppat.1012538. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39255320 Free PMC article.
-
A point mutation in GPI-attachment signal peptide accelerates the development of prion disease.Acta Neuropathol. 2023 May;145(5):637-650. doi: 10.1007/s00401-023-02553-5. Epub 2023 Mar 6. Acta Neuropathol. 2023. PMID: 36879070
-
A knockout cell library of GPI biosynthetic genes for functional studies of GPI-anchored proteins.Commun Biol. 2021 Jun 23;4(1):777. doi: 10.1038/s42003-021-02337-1. Commun Biol. 2021. PMID: 34162996 Free PMC article.
-
Siglec Signaling in the Tumor Microenvironment.Front Immunol. 2021 Dec 13;12:790317. doi: 10.3389/fimmu.2021.790317. eCollection 2021. Front Immunol. 2021. PMID: 34966391 Free PMC article. Review.
-
Cleavage site-directed antibodies reveal the prion protein in humans is shed by ADAM10 at Y226 and associates with misfolded protein deposits in neurodegenerative diseases.Acta Neuropathol. 2024 Jul 9;148(1):2. doi: 10.1007/s00401-024-02763-5. Acta Neuropathol. 2024. PMID: 38980441 Free PMC article.
References
-
- Ferguson M. A. J., Hart G. W., and Kinoshita T. (2015) in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds.), 3rd Ed., pp. 137–150, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

