Performance of a Modified Two-Tiered Testing Enzyme Immunoassay Algorithm for Serologic Diagnosis of Lyme Disease in Nova Scotia
- PMID: 32321781
- PMCID: PMC7315017
- DOI: 10.1128/JCM.01841-19
Performance of a Modified Two-Tiered Testing Enzyme Immunoassay Algorithm for Serologic Diagnosis of Lyme Disease in Nova Scotia
Abstract
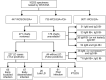
Compared to the standard two-tiered testing (STTT) algorithm for Lyme disease serology using an enzyme immunoassay (EIA) followed by Western blotting, data from the United States suggest that a modified two-tiered testing (MTTT) algorithm employing two EIAs has improved sensitivity to detect early localized Borrelia burgdorferi infections without compromising specificity. From 2011 to 2014, in the Canadian province of Nova Scotia, where Lyme disease is hyperendemic, sera submitted for Lyme disease testing were subjected to a whole-cell EIA, followed by C6 EIA and subsequently IgM and/or IgG immunoblots on sera with EIA-positive or equivocal results. Here, we evaluate the effectiveness of the MTTT algorithm compared to the STTT approach in a Nova Scotian population. Retrospective chart reviews were performed on patients testing positive with the whole-cell and C6 EIAs (i.e., the MTTT algorithm). Patients were classified as having Lyme disease if they had a positive STTT result, a negative STTT result but symptoms consistent with Lyme disease, or evidence of seroconversion on paired specimens. Of the 10,253 specimens tested for Lyme disease serology, 9,806 (95.6%) were negative. Of 447 patients who tested positive, 271 charts were available for review, and 227 were classified as patients with Lyme disease. The MTTT algorithm detected 25% more early infections with a specificity of 99.56% (99.41 to 99.68%) compared to the STTT. These are the first Canadian data to show that serology using a whole-cell sonicate EIA followed by a C6 EIA (MTTT) had improved sensitivity for detecting early B. burgdorferi infection with specificity similar to that of two-tiered testing using Western blots.
Keywords: Lyme disease; modified two-tiered test (MTTT); serology; specificity.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Public Health Agency of Canada. 14 August 2018. Surveillance of Lyme disease. https://www.canada.ca/en/public-health/services/diseases/lyme-disease/su.... 18 September 2019, access date.
-
- Gasmi S, Ogden NH, Lindsay LR, Burns S, Fleming S, Badcock J, Hanan S, Gaulin C, Leblanc MA, Russell C, Nelder M, Hobbs L, Graham-Derham S, Lachance L, Scott AN, Galanis E, Koffi JK. 2017. Surveillance for Lyme disease in Canada: 2009–2015. Can Commun Dis Rep 43:194–199. doi: 10.14745/ccdr.v43i10a01. - DOI - PMC - PubMed
-
- Leighton PA, Koffi KJ, Pelcat Y, Lindsay LR, Ogden NH. 2012. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J Appl Ecol 49:457–464. doi: 10.1111/j.1365-2664.2012.02112.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

